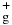ABSTRACT
Absolute vibrationally selected integral cross sections (σv+'s) for the ion–molecule reaction N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 have been measured by using the newly developed vacuum ultraviolet (VUV) laser pulsed field ionization-photoion (PFI-PI) double-quadrupole–double-octopole ion guide apparatus. By employing a novel electric field pulsing scheme to the VUV laser PFI-PI source, we have been able to prepare reactant N
; v+ = 0–2) + CH4 have been measured by using the newly developed vacuum ultraviolet (VUV) laser pulsed field ionization-photoion (PFI-PI) double-quadrupole–double-octopole ion guide apparatus. By employing a novel electric field pulsing scheme to the VUV laser PFI-PI source, we have been able to prepare reactant N ions in single-vibrational quantum states with not only high intensity and high purity but also high kinetic energy resolution, allowing integral cross section measurements to be conducted in the center-of-mass kinetic energies (Ecm's) from 0.05 to 10.00 eV. Three primary product channels corresponding to the formations of CH
ions in single-vibrational quantum states with not only high intensity and high purity but also high kinetic energy resolution, allowing integral cross section measurements to be conducted in the center-of-mass kinetic energies (Ecm's) from 0.05 to 10.00 eV. Three primary product channels corresponding to the formations of CH , CH
, CH , and N2H+ were identified. After correcting for the secondary reactions involving CH
, and N2H+ were identified. After correcting for the secondary reactions involving CH and CH
and CH , we have determined the σv+ values of the formation of these primary product ions, σv+(CH
, we have determined the σv+ values of the formation of these primary product ions, σv+(CH ), σv+(CH
), σv+(CH ), and σv+(N2H+), and their branching ratios, [σv+(CH
), and σv+(N2H+), and their branching ratios, [σv+(CH ): σv+(CH
): σv+(CH ): σv+(N2H+)]/σv+(CH
): σv+(N2H+)]/σv+(CH + CH
+ CH + N2H+), v+ = 0–2, in the Ecm range of 0.05–10.00 eV, where σv+(CH
+ N2H+), v+ = 0–2, in the Ecm range of 0.05–10.00 eV, where σv+(CH + CH
+ CH + N2H+) = σv+(CH
+ N2H+) = σv+(CH ) + σv+(CH
) + σv+(CH ) + σv+(N2H+). The branching ratios are found to be nearly independent of the v+ state and Ecm. Complex v+-state and Ecm dependences for σv+(CH
) + σv+(N2H+). The branching ratios are found to be nearly independent of the v+ state and Ecm. Complex v+-state and Ecm dependences for σv+(CH ), σv+(CH
), σv+(CH ), and σv+(N2H+) along with vibrational inhibition for the formation of these product ions are observed. The vibrational effects on the σv+ values are sufficiently large to warrant the inclusion of the vibrationally excited reactions N
), and σv+(N2H+) along with vibrational inhibition for the formation of these product ions are observed. The vibrational effects on the σv+ values are sufficiently large to warrant the inclusion of the vibrationally excited reactions N (X2Σ
(X2Σ ; v+ ⩾ 1) + CH4 for a more realistic modeling of the ion and neutral densities observed in the atmosphere of Titan. The cross-sectional data obtained in the present study are also useful for benchmarking theoretical calculations on ion–neutral collision dynamics.
; v+ ⩾ 1) + CH4 for a more realistic modeling of the ion and neutral densities observed in the atmosphere of Titan. The cross-sectional data obtained in the present study are also useful for benchmarking theoretical calculations on ion–neutral collision dynamics.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
The ion–molecule reaction of N + CH4 has been the subject of intense studies in the past few decades (Anicich et al. 2004; Gauglhofer & Kevan 1972; Gichuhi & Suits 2011; Gislason et al. 1969; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979; Wyatt et al. 1976). Besides the investigation of chemical reaction dynamics, an important motivation for the study of this reaction is that it represents one of the key initiating ion–molecule reactions for triggering the overall formation and chemical evolution of the atmosphere of Titan, which is the largest moon of Saturn and is recognized as one of the most Earth-like worlds found to date (Anicich et al. 2004; Anicich & McEwan 1997; Carrasco et al. 2008; McEwan et al. 1998; Suits 2009). The atmosphere of Titan is considered to be reminiscent of the primordial atmosphere of Earth (Suits 2009). Because of the implication concerning the origin of life on Earth, modeling and simulation of the chemical composition of Titan are needed for understanding the chemical evolution of its atmosphere (Anicich & McEwan 1997; Carrasco et al. 2008; Suits 2009). The atmospheric composition of Titan is known to consist of N2 (≈94%), CH4 (≈4%), and traces of H2, HCN, C2H2, C2H4, etc. The ionization of N2 by electron impact, solar vacuum ultraviolet (VUV), and cosmic rays (McEwan et al. 1998) constitutes the major source of N
+ CH4 has been the subject of intense studies in the past few decades (Anicich et al. 2004; Gauglhofer & Kevan 1972; Gichuhi & Suits 2011; Gislason et al. 1969; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979; Wyatt et al. 1976). Besides the investigation of chemical reaction dynamics, an important motivation for the study of this reaction is that it represents one of the key initiating ion–molecule reactions for triggering the overall formation and chemical evolution of the atmosphere of Titan, which is the largest moon of Saturn and is recognized as one of the most Earth-like worlds found to date (Anicich et al. 2004; Anicich & McEwan 1997; Carrasco et al. 2008; McEwan et al. 1998; Suits 2009). The atmosphere of Titan is considered to be reminiscent of the primordial atmosphere of Earth (Suits 2009). Because of the implication concerning the origin of life on Earth, modeling and simulation of the chemical composition of Titan are needed for understanding the chemical evolution of its atmosphere (Anicich & McEwan 1997; Carrasco et al. 2008; Suits 2009). The atmospheric composition of Titan is known to consist of N2 (≈94%), CH4 (≈4%), and traces of H2, HCN, C2H2, C2H4, etc. The ionization of N2 by electron impact, solar vacuum ultraviolet (VUV), and cosmic rays (McEwan et al. 1998) constitutes the major source of N . The mission of the Cassini–Huygens spacecraft sent to the Saturn system in 2004 (Owen 2005) has made available enhanced in situ data on the density profiles of ions and neutrals in the atmosphere of Titan, which should allow a more in depth simulation (Atreya 2007; Gu et al. 2009; Imanaka & Smith 2010; Israel et al. 2005; Niemann et al. 2005; Waite et al. 2007), and thus a better understanding of the chemical processes occurring on Titan. Such an effort would demand more detailed laboratory measurements of reaction rate constants or cross sections and product branching ratios for the relevant chemical reactions involved (Suits 2009).
. The mission of the Cassini–Huygens spacecraft sent to the Saturn system in 2004 (Owen 2005) has made available enhanced in situ data on the density profiles of ions and neutrals in the atmosphere of Titan, which should allow a more in depth simulation (Atreya 2007; Gu et al. 2009; Imanaka & Smith 2010; Israel et al. 2005; Niemann et al. 2005; Waite et al. 2007), and thus a better understanding of the chemical processes occurring on Titan. Such an effort would demand more detailed laboratory measurements of reaction rate constants or cross sections and product branching ratios for the relevant chemical reactions involved (Suits 2009).
Molecular ions such as N (X2Σ
(X2Σ ), produced by solar VUV and electron impact ionization can be formed in excited vibrational states, and the reactivity of these excited ions can be significantly different from that of the ground vibrational state. For example, the integral cross section of the N
), produced by solar VUV and electron impact ionization can be formed in excited vibrational states, and the reactivity of these excited ions can be significantly different from that of the ground vibrational state. For example, the integral cross section of the N (X2Σ
(X2Σ ; v+ = 0–2) + Ar charge transfer reaction has been shown to be greatly enhanced by vibrational excitation, with the integral cross sections for v+ = 1 and 2 to be about 100 and 200 fold greater than that for v+ = 0 (Chang et al. 2011, 2012). It is well known that vibrationally excited states of molecular ions in the ground electronic state that formed in this manner usually have long radiative lifetimes in the μs to ms range. For homonuclear diatomic species without a dipole moment, such as N
; v+ = 0–2) + Ar charge transfer reaction has been shown to be greatly enhanced by vibrational excitation, with the integral cross sections for v+ = 1 and 2 to be about 100 and 200 fold greater than that for v+ = 0 (Chang et al. 2011, 2012). It is well known that vibrationally excited states of molecular ions in the ground electronic state that formed in this manner usually have long radiative lifetimes in the μs to ms range. For homonuclear diatomic species without a dipole moment, such as N (X2Σ
(X2Σ ; v+ ⩾ 1), radiative decay to the ground vibrational state is not possible. Because of the long lifetimes, the reaction for N
; v+ ⩾ 1), radiative decay to the ground vibrational state is not possible. Because of the long lifetimes, the reaction for N in individual vibrational v+-states must be taken into account in a realistic modeling of the observed density profiles of electrons, ions, and neutrals in planetary ionospheres.
in individual vibrational v+-states must be taken into account in a realistic modeling of the observed density profiles of electrons, ions, and neutrals in planetary ionospheres.
To our knowledge, ion–molecule reactions involving vibrationally excited reactant N have not been considered in the modeling of ion and neutral density profiles observed in the atmosphere of Titan. The fact that the vibrational dependence of reaction cross sections has been neglected in the modeling of ion and neutral chemical compositions observed in planetary atmospheres, gas comae, and astrochemistry, can be ascribed to the lack of available state-selected cross-sectional data for relevant ion–molecule processes. Ion–neutral reactions generally occur in the temperature range of 300–5000 K in the ionospheres. Thus, for state-selected rate constants or cross sections to be useful for atmospheric modeling, they must be measured down to thermal energies or at center-of-mass collision energies of Ecm ≈ 30–40 meV. For Titan's atmosphere, one needs reaction cross sections (or rate coefficients) at much lower temperatures than 300 K. The demanding requirements for achieving fine control of both kinetic and internal energies for the reactant ions have not been achieved in previous state-selected experiments (Dressler et al. 2006; Ng 1992, 2002). Limited by the achievable kinetic energy resolution, the absolute integral cross sections obtained in previous vibrationally selected studies are limited to Ecm ≈ 0.5–1.0 eV, which is well above thermal energies.
have not been considered in the modeling of ion and neutral density profiles observed in the atmosphere of Titan. The fact that the vibrational dependence of reaction cross sections has been neglected in the modeling of ion and neutral chemical compositions observed in planetary atmospheres, gas comae, and astrochemistry, can be ascribed to the lack of available state-selected cross-sectional data for relevant ion–molecule processes. Ion–neutral reactions generally occur in the temperature range of 300–5000 K in the ionospheres. Thus, for state-selected rate constants or cross sections to be useful for atmospheric modeling, they must be measured down to thermal energies or at center-of-mass collision energies of Ecm ≈ 30–40 meV. For Titan's atmosphere, one needs reaction cross sections (or rate coefficients) at much lower temperatures than 300 K. The demanding requirements for achieving fine control of both kinetic and internal energies for the reactant ions have not been achieved in previous state-selected experiments (Dressler et al. 2006; Ng 1992, 2002). Limited by the achievable kinetic energy resolution, the absolute integral cross sections obtained in previous vibrationally selected studies are limited to Ecm ≈ 0.5–1.0 eV, which is well above thermal energies.
We have recently developed a molecular beam VUV laser pulsed-field ionization-photoion (PFI-PI) source and have successfully coupled this ion source to a double-quadrupole–double-octopole (DQDO) ion guide mass spectrometer for absolute integral cross-sectional measurements of ion–molecule reactions involving rovibrationally selected reactant ions (Chang et al. 2011, 2012; Xu et al. 2012). Employing this unique apparatus, we have obtained rovibrationally selected integral cross sections for the reactions, N (X2Σ
(X2Σ ; v+; N+) + Ar → Ar+ + N2 and H2O+(X2B1;
; v+; N+) + Ar → Ar+ + N2 and H2O+(X2B1;  ; N+Ka+Kc+) + D2 → H2DO+ + D in the Ecm range from thermal energies to 10.00 eV (Chang et al. 2011, 2012; Xu et al. 2012). The results of these experiments indicate that this ion–molecule reaction apparatus can be equally applicable for absolute cross-sectional measurements of other state-selected ion–molecule reactions involving atomic, diatomic, triatomic, and simple polyatomic ions of relevance to planetary atmospheres. This technical progress of achieving high internal state selectivity and high laboratory kinetic energy (Elab) resolution for the reactant ion beam presents a unique opportunity for a detailed examination of the kinetic, rotational, and vibrational energy dependencies on reaction cross sections of ion–molecule processes of importance occurring in planetary atmospheres and cometary comae.
; N+Ka+Kc+) + D2 → H2DO+ + D in the Ecm range from thermal energies to 10.00 eV (Chang et al. 2011, 2012; Xu et al. 2012). The results of these experiments indicate that this ion–molecule reaction apparatus can be equally applicable for absolute cross-sectional measurements of other state-selected ion–molecule reactions involving atomic, diatomic, triatomic, and simple polyatomic ions of relevance to planetary atmospheres. This technical progress of achieving high internal state selectivity and high laboratory kinetic energy (Elab) resolution for the reactant ion beam presents a unique opportunity for a detailed examination of the kinetic, rotational, and vibrational energy dependencies on reaction cross sections of ion–molecule processes of importance occurring in planetary atmospheres and cometary comae.
As early as 1969, Mahan et al. (Gislason et al. 1969) examined the dynamics of the N + CH4 reaction by the crossed ion–neutral beam method, from which the velocity distribution of the product ion N2H+ formed by H-atom transfer was measured, leading them to propose a stripping reaction model for the reaction process. Using the ion cyclotron resonance technique, Gauglhoffer et al. (Gauglhofer & Kevan 1972) have measured the rate constant for the reaction of N
+ CH4 reaction by the crossed ion–neutral beam method, from which the velocity distribution of the product ion N2H+ formed by H-atom transfer was measured, leading them to propose a stripping reaction model for the reaction process. Using the ion cyclotron resonance technique, Gauglhoffer et al. (Gauglhofer & Kevan 1972) have measured the rate constant for the reaction of N + CH4, which was found to be three times as large as the rate constant predicted by the Langevin–Gioumousis–Stevenson (LGS) model (Stevenson & Schissler 1958). Wyatt et al. (1976) examined the reaction of N
+ CH4, which was found to be three times as large as the rate constant predicted by the Langevin–Gioumousis–Stevenson (LGS) model (Stevenson & Schissler 1958). Wyatt et al. (1976) examined the reaction of N + CH4 → N2H+ + CH3 employing the ion beam-collision chamber method, from which the reaction cross sections as well as the velocity and angular distributions of product N2H+ were measured as a function of kinetic energy. Based on a selected ion flow tube study, Smith and co-workers (Smith et al. 1978) suggested that the N
+ CH4 → N2H+ + CH3 employing the ion beam-collision chamber method, from which the reaction cross sections as well as the velocity and angular distributions of product N2H+ were measured as a function of kinetic energy. Based on a selected ion flow tube study, Smith and co-workers (Smith et al. 1978) suggested that the N + CH4 reaction was mediated by charge transfer followed by ion fragmentation. In the latter experiment, only product CH
+ CH4 reaction was mediated by charge transfer followed by ion fragmentation. In the latter experiment, only product CH and CH
and CH ions were observed with a branching ratio of CH
ions were observed with a branching ratio of CH :CH
:CH = 0.93:0.07 and no H atom transfer reaction was detectable. A reaction rate constant of 1.0 × 10−9 cm3 s−1 was determined. In a later paper (Tichý et al. 1979) published by the same group, the CH
= 0.93:0.07 and no H atom transfer reaction was detectable. A reaction rate constant of 1.0 × 10−9 cm3 s−1 was determined. In a later paper (Tichý et al. 1979) published by the same group, the CH :CH
:CH branching ratio was obtained to be 0.89:0.11 and the rate constant was reported as 1.18 × 10−9 cm3 s−1. The N
branching ratio was obtained to be 0.89:0.11 and the rate constant was reported as 1.18 × 10−9 cm3 s−1. The N + CH4 reaction was also studied by Randeniya & Smith (1991) who employed a free jet flow reactor at very low temperatures (8–15 K), yielding a rate constant of 1.9 (±0.9) × 10−9 cm3 s−1, which was found to be independent of temperature in the latter temperature range. Furthermore, only two product channels for the formation of CH
+ CH4 reaction was also studied by Randeniya & Smith (1991) who employed a free jet flow reactor at very low temperatures (8–15 K), yielding a rate constant of 1.9 (±0.9) × 10−9 cm3 s−1, which was found to be independent of temperature in the latter temperature range. Furthermore, only two product channels for the formation of CH and CH
and CH with a branching ratio of (0.80 ± 0.10):(0.20 ± 0.10) at 10 K were observed. Employing an ion cyclotron resonance mass spectrometer, Anicich et al. (McEwan et al. 1998) also investigated the N
with a branching ratio of (0.80 ± 0.10):(0.20 ± 0.10) at 10 K were observed. Employing an ion cyclotron resonance mass spectrometer, Anicich et al. (McEwan et al. 1998) also investigated the N + CH4 reaction, from which three product channels, leading to the formation of CH
+ CH4 reaction, from which three product channels, leading to the formation of CH , CH
, CH , and N2H+, were observed with a branching ratio of 0.80:0.05:0.15, and an integral reaction rate constant of 1.14 × 10−9 cm3 s−1. Recently, Nicolas et al. (2003) measured the integral and differential cross sections of N
, and N2H+, were observed with a branching ratio of 0.80:0.05:0.15, and an integral reaction rate constant of 1.14 × 10−9 cm3 s−1. Recently, Nicolas et al. (2003) measured the integral and differential cross sections of N (v+ = 0) + CH4/CD4 in the Ecm range of 0.1–3.5 eV using a guided-ion beam ion–molecule reaction apparatus. Their results confirm the occurrence of three product channels that produce CH
(v+ = 0) + CH4/CD4 in the Ecm range of 0.1–3.5 eV using a guided-ion beam ion–molecule reaction apparatus. Their results confirm the occurrence of three product channels that produce CH , CH
, CH , and N2H+ ions with a branching ratio of 0.86:0.09:0.05, which was found to be independent of collision energies. Secondary reactions involving CH
, and N2H+ ions with a branching ratio of 0.86:0.09:0.05, which was found to be independent of collision energies. Secondary reactions involving CH and CH
and CH in the reaction gas cell were observed, and partial corrections on the integral cross sections to the product channels due to secondary reactions were made based on the rate data reported by Anicich & McEwan (1997). However, important secondary reactions, which may affect the integral cross sections and the branch ratio, were ignored in their data analysis. In 2004, V. G. Anicich and co-workers (Anicich et al. 2004) reexamined the reaction of N
in the reaction gas cell were observed, and partial corrections on the integral cross sections to the product channels due to secondary reactions were made based on the rate data reported by Anicich & McEwan (1997). However, important secondary reactions, which may affect the integral cross sections and the branch ratio, were ignored in their data analysis. In 2004, V. G. Anicich and co-workers (Anicich et al. 2004) reexamined the reaction of N + CH4, and only two reaction channels leading to the formation of CH
+ CH4, and only two reaction channels leading to the formation of CH and CH
and CH ions were observed. The branching ratio and rate constant were determined as 0.88: 0.12 and 1.00 × 10−9 cm3 s−1, respectively. More recently, Gichuhi & Suits (2011) examined the low-temperature (≈45 K) primary branching ratio of N
ions were observed. The branching ratio and rate constant were determined as 0.88: 0.12 and 1.00 × 10−9 cm3 s−1, respectively. More recently, Gichuhi & Suits (2011) examined the low-temperature (≈45 K) primary branching ratio of N + CH4 reaction in a free jet by preparing N
+ CH4 reaction in a free jet by preparing N (X2Σ
(X2Σ ; v = 0) via a 2 + 1 resonance-enhanced multiphoton ionization scheme. Only two reaction channels for CH
; v = 0) via a 2 + 1 resonance-enhanced multiphoton ionization scheme. Only two reaction channels for CH and CH
and CH ions were observed, and a corresponding branch ratio of 0.83:0.17 was reported.
ions were observed, and a corresponding branch ratio of 0.83:0.17 was reported.
To our knowledge, the reactivity of N (X2Σ
(X2Σ ; v+ ⩾ 1) with CH4 has not been examined previously. Using the newly established VUV laser PFI-PI DQDO ion guide apparatus, we have performed a detailed measurement of the vibrationally selected reaction of N
; v+ ⩾ 1) with CH4 has not been examined previously. Using the newly established VUV laser PFI-PI DQDO ion guide apparatus, we have performed a detailed measurement of the vibrationally selected reaction of N (X2Σ
(X2Σ ; v+ = 0, 1, and 2) + CH4. In this report, we present the vibrationally selected absolute integral cross sections (σv+'s) for the formation of product CH
; v+ = 0, 1, and 2) + CH4. In this report, we present the vibrationally selected absolute integral cross sections (σv+'s) for the formation of product CH , CH
, CH , and N2H+ ions as well as the branching ratios, [σv+(CH
, and N2H+ ions as well as the branching ratios, [σv+(CH ): σv+(CH
): σv+(CH ): σv+(N2H+)]/σv+(CH
): σv+(N2H+)]/σv+(CH + CH
+ CH + N2H+), for v+ = 0, 1, and 2, in the Ecm range of 0.05–10.0 eV, where σv+(CH
+ N2H+), for v+ = 0, 1, and 2, in the Ecm range of 0.05–10.0 eV, where σv+(CH + CH
+ CH + N2H+) = σv+(CH
+ N2H+) = σv+(CH ) + σv+(CH
) + σv+(CH ) + σv+(N2H+).
) + σv+(N2H+).
2. EXPERIMENTAL CONSIDERATION
The present measurement of the absolute integral cross sections for the vibrationally selected N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 reaction was conducted using the VUV laser PFI-PI DQDO apparatus, which has been reported in detail previously (Chang et al. 2011, 2012; Xu et al. 2012). Briefly, the apparatus consists of a tunable VUV laser system, a PFI-PI ion source, and a DQDO ion guide mass spectrometer. Tunable VUV laser radiation is generated by four-wave sum-frequency (2v1 + v2) mixing using a Kr pulsed jet as the nonlinear medium, where v1 and v2 represent the respective ultraviolet (UV) and visible (VIS) outputs of two dye lasers, which were pumped by the second and third harmonic outputs of one identical Nd:YAG laser operated at 15 Hz. The N2 molecules were introduced into the photoionization/photoexcitation (PI/PEX) region of the ion source in the form of a supersonic molecular beam traveling along the central axis of the DQDO mass spectrometer. The vibrationally selected N
; v+ = 0–2) + CH4 reaction was conducted using the VUV laser PFI-PI DQDO apparatus, which has been reported in detail previously (Chang et al. 2011, 2012; Xu et al. 2012). Briefly, the apparatus consists of a tunable VUV laser system, a PFI-PI ion source, and a DQDO ion guide mass spectrometer. Tunable VUV laser radiation is generated by four-wave sum-frequency (2v1 + v2) mixing using a Kr pulsed jet as the nonlinear medium, where v1 and v2 represent the respective ultraviolet (UV) and visible (VIS) outputs of two dye lasers, which were pumped by the second and third harmonic outputs of one identical Nd:YAG laser operated at 15 Hz. The N2 molecules were introduced into the photoionization/photoexcitation (PI/PEX) region of the ion source in the form of a supersonic molecular beam traveling along the central axis of the DQDO mass spectrometer. The vibrationally selected N (X2Σ
(X2Σ ; v+ = 0–2) PFI-PI ions were prepared by employing a novel electric-field pulsing scheme on the VUV laser PFI-PI ion source for retarding the prompt ions produced by direct VUV photoionization, generating the PFI-PI ions from high-n (n ≈ 100) Rydberg N2*(n) species formed by VUV excitation of the N2 molecular beam, and extracting the ions thus produced from the ion source. The control of the timing and magnitudes of the pulsed electric fields applied allows for the preparation of an N
; v+ = 0–2) PFI-PI ions were prepared by employing a novel electric-field pulsing scheme on the VUV laser PFI-PI ion source for retarding the prompt ions produced by direct VUV photoionization, generating the PFI-PI ions from high-n (n ≈ 100) Rydberg N2*(n) species formed by VUV excitation of the N2 molecular beam, and extracting the ions thus produced from the ion source. The control of the timing and magnitudes of the pulsed electric fields applied allows for the preparation of an N ion beam with not only high internal state purity and high intensity but also high kinetic energy resolution. The DQDO ion guide mass spectrometer consists of two quadrupole mass spectrometers (QMSs), two radio frequency (rf) octopole ion guides, and reaction gas cells, where the N
ion beam with not only high internal state purity and high intensity but also high kinetic energy resolution. The DQDO ion guide mass spectrometer consists of two quadrupole mass spectrometers (QMSs), two radio frequency (rf) octopole ion guides, and reaction gas cells, where the N + CH4 reaction occurs. The reactant QMS is designed for mass selection of reactant ions to enter the first octopole ion guide. Since only N
+ CH4 reaction occurs. The reactant QMS is designed for mass selection of reactant ions to enter the first octopole ion guide. Since only N PFI-PIs are generated and selected by the PFI-PI scheme in the present experiment, the reactant QMS was operated as an ion lens to guide the reactant N
PFI-PIs are generated and selected by the PFI-PI scheme in the present experiment, the reactant QMS was operated as an ion lens to guide the reactant N PFI-PIs into the reaction gas cell. The pressure of the neutral reactant CH4 in the gas cell is monitored by an MKS Baratron. The first and second rf-octopole ion guides are designed to join at the middle of the reaction gas cell. While the same rf was applied to both the first and second octopoles to confine and guide all reactant and product ions into the product QMS for detection by the ion detector, different dc voltages could be applied to the first and second octopoles, serving to extract slow product ions from the gas cell, and thus minimizing secondary reactions. The ion detector has the design of a Daley detector except that the photomultiplier tube was replaced by a dual set of microchannel plates. The ion signals passed through an amplifier and a discriminator prior to be measured by a multichannel scalar (MCS).
PFI-PIs into the reaction gas cell. The pressure of the neutral reactant CH4 in the gas cell is monitored by an MKS Baratron. The first and second rf-octopole ion guides are designed to join at the middle of the reaction gas cell. While the same rf was applied to both the first and second octopoles to confine and guide all reactant and product ions into the product QMS for detection by the ion detector, different dc voltages could be applied to the first and second octopoles, serving to extract slow product ions from the gas cell, and thus minimizing secondary reactions. The ion detector has the design of a Daley detector except that the photomultiplier tube was replaced by a dual set of microchannel plates. The ion signals passed through an amplifier and a discriminator prior to be measured by a multichannel scalar (MCS).
All cross-sectional data presented in this study are based on at least three independent and reproducible measurements. The standard deviations (generally about 10%) are determined by the reproducibility of the independent measurements. However, the systematic uncertainties for the experimental cross sections presented here are estimated to be 30% (Chang et al. 2011). The N2 and CH4 gases used in this experiment have purities of 99.998% and 99.999%, respectively.
3. RESULTS AND DISCUSSIONS
Based on the energetic analysis (Lias et al. 1988), four possible exothermic reaction channels leading to the formation of CH , CH
, CH , CH
, CH , and N2H+ from the N
, and N2H+ from the N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 collisions are shown as reactions (1)–(4):
; v+ = 0–2) + CH4 collisions are shown as reactions (1)–(4):
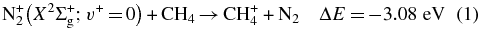
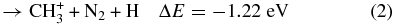
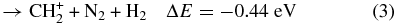
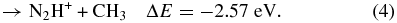
Here, the exothermicities (−ΔE values) for the reactions associated with reactant N (X2Σ
(X2Σ ; v+ = 0) are shown in reactions (1)–(4). The exothermicities for these reactions with N
; v+ = 0) are shown in reactions (1)–(4). The exothermicities for these reactions with N (X2Σ
(X2Σ ) prepared in the v+ = 1 and two vibrational states are higher by 0.271 and 0.535 eV, respectively (Chang et al. 2012).
) prepared in the v+ = 1 and two vibrational states are higher by 0.271 and 0.535 eV, respectively (Chang et al. 2012).
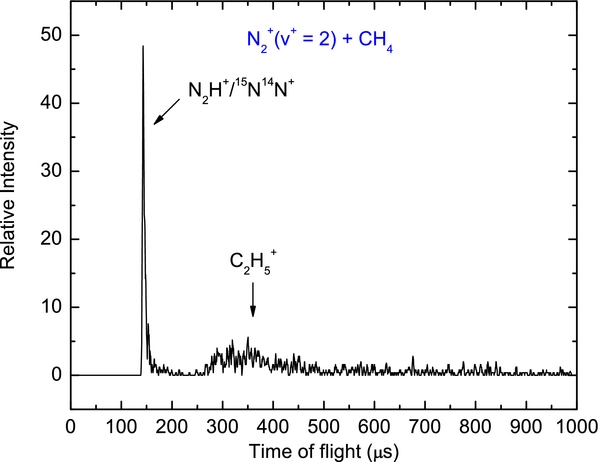
Figure 1(a) depicts the mass spectrum for the vibrationally selected N (X2Σ
(X2Σ ; v+ = 0) ion beam at Ecm = 0.30 eV by using the product QMS. Since this spectrum was obtained without CH4 in the reaction gas cell, only the reactant ions were observed. The two ion peaks observed at m/z = 28 and 29 can thus be assigned as the 14N14N+ and 15N14N+ ions, respectively. The ratio of the two ion peaks found in Figure 1(a) is consistent with the natural isotopic distribution of 14N14N+ and 15N14N+. When reactant neutral CH4 was filled into the gas cell at a pressure of 4 × 10−5 Torr, we recorded the mass spectrum shown in Figure 1(b), in which four ion peaks at m/z = 14, 15, 28, and 29 were identified. The ion peaks at m/z = 14 and 15 can be unambiguously assigned as CH
; v+ = 0) ion beam at Ecm = 0.30 eV by using the product QMS. Since this spectrum was obtained without CH4 in the reaction gas cell, only the reactant ions were observed. The two ion peaks observed at m/z = 28 and 29 can thus be assigned as the 14N14N+ and 15N14N+ ions, respectively. The ratio of the two ion peaks found in Figure 1(a) is consistent with the natural isotopic distribution of 14N14N+ and 15N14N+. When reactant neutral CH4 was filled into the gas cell at a pressure of 4 × 10−5 Torr, we recorded the mass spectrum shown in Figure 1(b), in which four ion peaks at m/z = 14, 15, 28, and 29 were identified. The ion peaks at m/z = 14 and 15 can be unambiguously assigned as CH and CH
and CH ions, formed by reactions (3) and (2), respectively. The ion peaks at m/z = 28 must have the major contribution from N
ions, formed by reactions (3) and (2), respectively. The ion peaks at m/z = 28 must have the major contribution from N ; and the ion peak at m/z = 29 should be contributed by 15N14N+ and N2H+ formed by the H atom transfer reaction (4). The interference for the detection of product N2H+ by the 15N14N+ background can be easily removed by subtracting the corresponding ion intensities of the m/z = 29 ions observed with and without the CH4 reactant gas filled in the gas cell. Another method to eliminate the interference of 15N14N+ would be to set the reactant QMS to transmit only the 14N14N+ ions. The mass spectrum of Figure 1(b) clearly shows that CH
; and the ion peak at m/z = 29 should be contributed by 15N14N+ and N2H+ formed by the H atom transfer reaction (4). The interference for the detection of product N2H+ by the 15N14N+ background can be easily removed by subtracting the corresponding ion intensities of the m/z = 29 ions observed with and without the CH4 reactant gas filled in the gas cell. Another method to eliminate the interference of 15N14N+ would be to set the reactant QMS to transmit only the 14N14N+ ions. The mass spectrum of Figure 1(b) clearly shows that CH , CH
, CH , and N2H+ are primary product ions of the reaction of N
, and N2H+ are primary product ions of the reaction of N (X2Σ
(X2Σ ; v+ = 0) + CH4. Furthermore, no CH
; v+ = 0) + CH4. Furthermore, no CH ions associated with the charge-transfer reaction mechanism are observed. This latter observation is consistent with the results of all previous experimental investigations (Anicich et al. 2004; Gauglhofer & Kevan 1972; Gichuhi & Suits 2011; Gislason et al. 1969; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979; Wyatt et al. 1976).
ions associated with the charge-transfer reaction mechanism are observed. This latter observation is consistent with the results of all previous experimental investigations (Anicich et al. 2004; Gauglhofer & Kevan 1972; Gichuhi & Suits 2011; Gislason et al. 1969; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979; Wyatt et al. 1976).
Figure 1. (a) Mass spectrum for the vibrationally selected N (X2Σ
(X2Σ ; v+ = 0) ion beam at Ecm = 0.30 eV recorded using the product QMS. This spectrum was obtained without CH4 in the reaction gas cell. The two ion peaks observed at m/z = 28 and 29 are assigned as the 14N14N+ and 15N14N+ ions, respectively. (b) Mass spectrum observed when reactant neutral CH4 was filled into the gas cell at a pressure of 4 × 10−5 Torr, i.e., the mass spectrum results from the N
; v+ = 0) ion beam at Ecm = 0.30 eV recorded using the product QMS. This spectrum was obtained without CH4 in the reaction gas cell. The two ion peaks observed at m/z = 28 and 29 are assigned as the 14N14N+ and 15N14N+ ions, respectively. (b) Mass spectrum observed when reactant neutral CH4 was filled into the gas cell at a pressure of 4 × 10−5 Torr, i.e., the mass spectrum results from the N (X2Σ
(X2Σ ; v+ = 0) + CH4 at Ecm = 0.30 eV. Four ion peaks observed at m/z = 14, 15, 28, and 29 are assigned as CH
; v+ = 0) + CH4 at Ecm = 0.30 eV. Four ion peaks observed at m/z = 14, 15, 28, and 29 are assigned as CH , CH
, CH , N
, N /C2H
/C2H , and N2H+/15N14N+, respectively. No CH
, and N2H+/15N14N+, respectively. No CH ions are observed.
ions are observed.
Download figure:
Standard image High-resolution imageThe use of a low gas cell pressure (4 × 10−5 Torr) of reactant CH4 was intended to minimize secondary reactions involving products CH and CH
and CH with CH4 in the reaction gas cell. Nevertheless, secondary reactions of CH
with CH4 in the reaction gas cell. Nevertheless, secondary reactions of CH and CH
and CH according to reactions (5)–(7) are still found to occur.
according to reactions (5)–(7) are still found to occur.
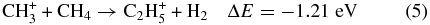
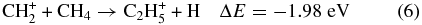
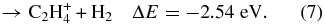
Previous studies have shown that these exothermic reactions, possibly proceeding with a complex formation mechanism at thermal energies, have very high reaction rate constants. Anicich & McEwan (1997) have shown that C2H can be formed efficiently by the reaction between CH
can be formed efficiently by the reaction between CH and CH4 and thus, the observed m/z = 28 ion peak should include the contribution from C2H
and CH4 and thus, the observed m/z = 28 ion peak should include the contribution from C2H ions. As shown below, the formation of slow C2H
ions. As shown below, the formation of slow C2H ions by this secondary reaction (7) can be distinguished by the time-of-flight (TOF) method. The minor ion peak appearing at m/z = 29 is also expected to have an attribution by secondary C2H
ions by this secondary reaction (7) can be distinguished by the time-of-flight (TOF) method. The minor ion peak appearing at m/z = 29 is also expected to have an attribution by secondary C2H ions produced by reactions (5) and (6) (Anicich & McEwan 1997). Secondary C2H
ions produced by reactions (5) and (6) (Anicich & McEwan 1997). Secondary C2H ions can also be distinguished from primary N2H+ ions by the TOF method as illustrated below.
ions can also be distinguished from primary N2H+ ions by the TOF method as illustrated below.
The occurrence of secondary reactions (5)–(7) has the effect of reducing the apparent cross sections for the formation of CH and CH
and CH , and increasing those for N2H+ if proper corrections for secondary reactions are not made. In order to determine accurate integral cross sections and branching ratios for the formation of CH
, and increasing those for N2H+ if proper corrections for secondary reactions are not made. In order to determine accurate integral cross sections and branching ratios for the formation of CH , CH
, CH , and N2H+, we have examined the secondary reactions of CH
, and N2H+, we have examined the secondary reactions of CH and CH
and CH in detail. At similar experimental conditions, Nicolas et al. have verified the occurrence of secondary reactions between CH3+ (CH
in detail. At similar experimental conditions, Nicolas et al. have verified the occurrence of secondary reactions between CH3+ (CH ) and CH4 to form C2H
) and CH4 to form C2H by using deuterated methane CD4 as the neutral reactant gas (Nicolas et al. 2003). Since the reaction mechanisms for the formation of N2H+ and C2H
by using deuterated methane CD4 as the neutral reactant gas (Nicolas et al. 2003). Since the reaction mechanisms for the formation of N2H+ and C2H ions are different, they are produced with different velocity distributions. This allows N2H+ and C2H
ions are different, they are produced with different velocity distributions. This allows N2H+ and C2H to be separated and detected by the TOF method. The N2H+ ions are known to be formed by the reaction of N
to be separated and detected by the TOF method. The N2H+ ions are known to be formed by the reaction of N + CH4 via a direct H-stripping mechanism. Due to the significantly lighter mass of H atoms compared to that of N
+ CH4 via a direct H-stripping mechanism. Due to the significantly lighter mass of H atoms compared to that of N ions, the velocities of the N2H+ ions are expected to be similar to those of N
ions, the velocities of the N2H+ ions are expected to be similar to those of N ions. According to the charge transfer mechanism for the reaction of N
ions. According to the charge transfer mechanism for the reaction of N + CH4, CH
+ CH4, CH and CH
and CH are fragments of excited CH
are fragments of excited CH formed by charge transfer collisions of N
formed by charge transfer collisions of N and CH4. Considering that the charge transfer CH
and CH4. Considering that the charge transfer CH ions are formed mostly at thermal energies, the velocities of secondary C2H
ions are formed mostly at thermal energies, the velocities of secondary C2H and C2H
and C2H ions produced by secondary reactions of CH
ions produced by secondary reactions of CH and CH
and CH fragments from CH
fragments from CH are also expected to be slow. This expectation can be verified by TOF measurements of the mass 28 (mass 29) ions resulting from the N
are also expected to be slow. This expectation can be verified by TOF measurements of the mass 28 (mass 29) ions resulting from the N (X2Σ
(X2Σ ; v+) + CH4 reaction by using the MCS and setting the product QMS at m/z = 28 (m/z = 29).
; v+) + CH4 reaction by using the MCS and setting the product QMS at m/z = 28 (m/z = 29).
Figure 2 depicts the TOF spectrum of mass 29 ions resulting from the N (X2Σ
(X2Σ ; v+ = 2) + CH4 reaction at Ecm = 0.5 eV. The prominent sharp ion peak appearing at about 150 μs is assigned to N2H+/15N14N+ ions. As pointed out above, the interference of 15N14N+ in the measurement of product N2H+ can be easily corrected by taking the difference between the mass 29 ion intensities observed with and without the reactant CH4 gas filled in the reaction gas cell. The broad ion TOF peak observed at 260 μs with a long tail extending to about 1000 μs is attributed to C2H
; v+ = 2) + CH4 reaction at Ecm = 0.5 eV. The prominent sharp ion peak appearing at about 150 μs is assigned to N2H+/15N14N+ ions. As pointed out above, the interference of 15N14N+ in the measurement of product N2H+ can be easily corrected by taking the difference between the mass 29 ion intensities observed with and without the reactant CH4 gas filled in the reaction gas cell. The broad ion TOF peak observed at 260 μs with a long tail extending to about 1000 μs is attributed to C2H ions produced by the secondary reactions (5) and (6). Based on this assignment, the intensity of C2H
ions produced by the secondary reactions (5) and (6). Based on this assignment, the intensity of C2H ions can be measured by summing all the ion counts of the broad peak covering the region of 260–1000 μs for a fixed accumulation time of the TOF spectrum.
ions can be measured by summing all the ion counts of the broad peak covering the region of 260–1000 μs for a fixed accumulation time of the TOF spectrum.
Figure 2. TOF spectrum of mass 29 ions resulting from the N (X2Σ
(X2Σ ; v+ = 2) + CH4 reaction at Ecm = 0.50 eV recorded by using the MCS and setting the product QMS at m/z = 29. The accumulation time for this spectrum is 300 s. The sharp ion peak at about 150 μs is attributed to N2H+/15N14N+ ions, which are expected to have high kinetic energies. The slow ions associated with the broad ion peak extending from 250 μs to ≈1000 μs are attributed to C2H
; v+ = 2) + CH4 reaction at Ecm = 0.50 eV recorded by using the MCS and setting the product QMS at m/z = 29. The accumulation time for this spectrum is 300 s. The sharp ion peak at about 150 μs is attributed to N2H+/15N14N+ ions, which are expected to have high kinetic energies. The slow ions associated with the broad ion peak extending from 250 μs to ≈1000 μs are attributed to C2H ions, which are produced from the secondary reactions of CH
ions, which are produced from the secondary reactions of CH and CH
and CH ions with CH4.
ions with CH4.
Download figure:
Standard image High-resolution imageThe measured TOF spectrum for the mass 28 ions resulting from the N (X2Σ
(X2Σ ; v+ = 0) + CH4 reaction at Ecm = 0.5 eV as shown in Figure 3(b) reveals a sharp peak at about 150 μs and a broad peak at around 300 μs with a long tail extending up to more than 1000 μs. As a comparison, we have recorded the TOF spectrum (Figure 3(a)) by shutting off the neutral reactant CH4 gas from entering the reaction gas cell. As expected, the latter TOF spectrum shows a single sharp peak for reactant N
; v+ = 0) + CH4 reaction at Ecm = 0.5 eV as shown in Figure 3(b) reveals a sharp peak at about 150 μs and a broad peak at around 300 μs with a long tail extending up to more than 1000 μs. As a comparison, we have recorded the TOF spectrum (Figure 3(a)) by shutting off the neutral reactant CH4 gas from entering the reaction gas cell. As expected, the latter TOF spectrum shows a single sharp peak for reactant N appearing at 150 μs with nearly no ion counts at TOFs longer than 200 μs. Thus, the sharp peak at 150 μs and the broad peak at 300 μs can be ascribed to unreacted N
appearing at 150 μs with nearly no ion counts at TOFs longer than 200 μs. Thus, the sharp peak at 150 μs and the broad peak at 300 μs can be ascribed to unreacted N ions and secondary C2H
ions and secondary C2H ions, respectively. The seeming weakness of this broad peak for C2H
ions, respectively. The seeming weakness of this broad peak for C2H compared to that for C2H
compared to that for C2H in Figure 2 is due to the significantly shorter time (30 s) used in the accumulation of the spectrum of Figure 3(b) compared to the accumulation time (300 s) for the spectrum of Figure 2. Nevertheless, the observation of the broad peak for C2H
in Figure 2 is due to the significantly shorter time (30 s) used in the accumulation of the spectrum of Figure 3(b) compared to the accumulation time (300 s) for the spectrum of Figure 2. Nevertheless, the observation of the broad peak for C2H can be taken as support for the occurrence of reaction (7) in the reaction gas cell.
can be taken as support for the occurrence of reaction (7) in the reaction gas cell.
Figure 3. TOF spectrum of mass 28 ions originating from the N (X2Σ
(X2Σ ; v+ = 2) + CH4 reaction at Ecm = 0.50 eV recorded by using the MCS and setting the QMS at m/z = 28. The respective TOF spectra of (a) and (b) were obtained without and with the neutral CH4 gas filled into the reaction gas cell. The accumulation time for each TOF spectrum is 30 s. The TOF spectrum of panel (a) is featured by the single sharp peak at ≈150 μs corresponding to reactant N
; v+ = 2) + CH4 reaction at Ecm = 0.50 eV recorded by using the MCS and setting the QMS at m/z = 28. The respective TOF spectra of (a) and (b) were obtained without and with the neutral CH4 gas filled into the reaction gas cell. The accumulation time for each TOF spectrum is 30 s. The TOF spectrum of panel (a) is featured by the single sharp peak at ≈150 μs corresponding to reactant N , whereas the TOF spectrum of panel (b) reveals a weak, broad peak extending from 180 to more than 1000 μs, which is associated with the formation of secondary C2H
, whereas the TOF spectrum of panel (b) reveals a weak, broad peak extending from 180 to more than 1000 μs, which is associated with the formation of secondary C2H ions, in addition to the unreacted reactant N
ions, in addition to the unreacted reactant N peak.
peak.
Download figure:
Standard image High-resolution imageIn order to make the proper corrections of secondary reactions in the determination of the absolute integral cross sections, we need to partition the measured C2H intensity to contributions from the CH
intensity to contributions from the CH + CH4 and CH
+ CH4 and CH + CH4 reactions. Here, we have adopted the argument from Nicolas et al. (2003) by assigning the relative contributions from these reactions according to the ratio of their known thermal rate constants, k(CH
+ CH4 reactions. Here, we have adopted the argument from Nicolas et al. (2003) by assigning the relative contributions from these reactions according to the ratio of their known thermal rate constants, k(CH ) and k(CH
) and k(CH ), which are reasonably well determined to be =1.1 × 10−9 cm3 s−1 and 3.9 × 10−9 cm3 s−1, respectively. The application of this approach here is based on the crude assumption that the ratio k(CH
), which are reasonably well determined to be =1.1 × 10−9 cm3 s−1 and 3.9 × 10−9 cm3 s−1, respectively. The application of this approach here is based on the crude assumption that the ratio k(CH )/k(CH
)/k(CH ) is constant for CH
) is constant for CH and CH
and CH formed in the N
formed in the N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 reactions at the Ecm range of interest in the present experiment. In Nicolas et al. (2003), the authors assumed that the CH
; v+ = 0–2) + CH4 reactions at the Ecm range of interest in the present experiment. In Nicolas et al. (2003), the authors assumed that the CH + CH4 reaction only produced C2H
+ CH4 reaction only produced C2H and the production of C2H
and the production of C2H was not included in their consideration. The branching ratio for reactions (6) and (7) at thermal energies was determined to be 0.3:0.7 by Anicich & McEwan (1997), indicating that more CH
was not included in their consideration. The branching ratio for reactions (6) and (7) at thermal energies was determined to be 0.3:0.7 by Anicich & McEwan (1997), indicating that more CH ions undergo reaction (7) to generate C2H
ions undergo reaction (7) to generate C2H ion than producing C2H
ion than producing C2H by reaction (6). Combining the measured intensities of C2H
by reaction (6). Combining the measured intensities of C2H , the rate constant ratio k(CH
, the rate constant ratio k(CH )/k(CH
)/k(CH ), and the branching ratio for secondary reactions (6) and (7), we have been able to determine the proportions of primary CH
), and the branching ratio for secondary reactions (6) and (7), we have been able to determine the proportions of primary CH and CH
and CH ions that have undergone secondary reactions (5)–(7). These corrections, together with the intensities of the CH
ions that have undergone secondary reactions (5)–(7). These corrections, together with the intensities of the CH , CH
, CH , and N2H+ ions measured directly, are used for the determination of the σv+(CH
, and N2H+ ions measured directly, are used for the determination of the σv+(CH ), σv+(CH
), σv+(CH ), and σv+(N2H+) values and their branching ratios, [σv+(CH
), and σv+(N2H+) values and their branching ratios, [σv+(CH ):σv+(CH
):σv+(CH ):σv+(N2H+)]/σv+(CH
):σv+(N2H+)]/σv+(CH + CH
+ CH + N2H+), for v+ = 0, 1, and 2.
+ N2H+), for v+ = 0, 1, and 2.
Instead of using the branching ratio of 0.3:0.7 determined by Anicich & McEwan (1997) to estimate the intensity of C2H relative to the measured C2H
relative to the measured C2H intensity, the intensity of C2H
intensity, the intensity of C2H can be measured directly by the TOF method as shown in the spectrum of Figure 3(b). However, due to the overwhelming intensity of the N
can be measured directly by the TOF method as shown in the spectrum of Figure 3(b). However, due to the overwhelming intensity of the N TOF peak, the broad peak assigned to C2H
TOF peak, the broad peak assigned to C2H was found to overlap with the tail of the reactant N
was found to overlap with the tail of the reactant N peak for the N
peak for the N + CH4 reaction occurring at Ecm < 0.50 eV. Since this introduces an error in the measurement of the C2H
+ CH4 reaction occurring at Ecm < 0.50 eV. Since this introduces an error in the measurement of the C2H , we have decided to use the above scheme of relying on the branching ratio for reactions (6) and (7) to estimate the contribution of the C2H
, we have decided to use the above scheme of relying on the branching ratio for reactions (6) and (7) to estimate the contribution of the C2H intensity. We have compared the branching ratios deduced by the two approaches at Ecm = 0.50 and 1.00 eV and found that the observed discrepancies are within the assigned uncertainty (10%–15%) for the branching ratio measurements.
intensity. We have compared the branching ratios deduced by the two approaches at Ecm = 0.50 and 1.00 eV and found that the observed discrepancies are within the assigned uncertainty (10%–15%) for the branching ratio measurements.
After making the corrections due to secondary reactions, the vibrationally selected absolute integral cross sections, σv+(CH ), σv+(CH
), σv+(CH ), and σv+(N2H+) determined in the v+ range of 0–2 and the Ecm range of 0.05–10.00 eV are plotted in Figures 4(a)–(c), respectively, to illustrate the vibrational and Ecm effects. For all Ecm values examined in the present study, the absolute integral reaction cross sections for the formation of CH
), and σv+(N2H+) determined in the v+ range of 0–2 and the Ecm range of 0.05–10.00 eV are plotted in Figures 4(a)–(c), respectively, to illustrate the vibrational and Ecm effects. For all Ecm values examined in the present study, the absolute integral reaction cross sections for the formation of CH , CH
, CH , and N2H+ at a given Ecm are in the order: σv+(CH
, and N2H+ at a given Ecm are in the order: σv+(CH ) > σv+(CH
) > σv+(CH ) > σv+(N2H+), v+ = 0, 1, and 2. The integral cross sections of all three product channels are found to increase as Ecm is decreased. This trend is consistent with the exothermic nature of these reactions and the prediction of the LGS model (Stevenson & Schissler 1958). We note that the Ecm dependences for σv+(CH
) > σv+(N2H+), v+ = 0, 1, and 2. The integral cross sections of all three product channels are found to increase as Ecm is decreased. This trend is consistent with the exothermic nature of these reactions and the prediction of the LGS model (Stevenson & Schissler 1958). We note that the Ecm dependences for σv+(CH ) and σv+(CH
) and σv+(CH ), v+ = 0–2, at Ecm = 0.30–10.00 eV are similar. This observation is consistent with the previous suggestion that the formation of CH
), v+ = 0–2, at Ecm = 0.30–10.00 eV are similar. This observation is consistent with the previous suggestion that the formation of CH and CH
and CH is governed by a common dissociative charge transfer mechanism.
is governed by a common dissociative charge transfer mechanism.
Figure 4. Absolute integral cross sections for the formations of (a) CH [σv+(CH
[σv+(CH )], (b) CH
)], (b) CH [σv+(CH
[σv+(CH )], and (c) N2H+ [σv+(N2H+)] from the vibrationally state-selected ion–molecule reaction N
)], and (c) N2H+ [σv+(N2H+)] from the vibrationally state-selected ion–molecule reaction N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 obtained in the Ecm range of 0.05–10.00 eV. The standard deviations (≈10%) show the reproducibility of independent measurements.
; v+ = 0–2) + CH4 obtained in the Ecm range of 0.05–10.00 eV. The standard deviations (≈10%) show the reproducibility of independent measurements.
Download figure:
Standard image High-resolution imageAs shown in Figures 4(a)–(c), complicated vibrational dependences are observed by comparing the σv+(CH ), σv+(CH
), σv+(CH ), and σv+(N2H+) values obtained for v+ = 0, 1, and 2. In the Ecm range of 0.05–0.40 eV, the vibrationally selected absolute integral cross sections are found to be on the order of σ0 > σ2 > σ1 for the formation of CH
), and σv+(N2H+) values obtained for v+ = 0, 1, and 2. In the Ecm range of 0.05–0.40 eV, the vibrationally selected absolute integral cross sections are found to be on the order of σ0 > σ2 > σ1 for the formation of CH and N2H+. The σ1(CH
and N2H+. The σ1(CH ) [σ1(N2H+)] is ≈25% (≈40%) lower than σ0(CH
) [σ1(N2H+)] is ≈25% (≈40%) lower than σ0(CH ) [σ0(N2H+)]. That is, the N
) [σ0(N2H+)]. That is, the N (X2Σ
(X2Σ ) + CH4 collisions to produce CH
) + CH4 collisions to produce CH and N2H+ are significantly inhibited by vibrational excitation of N
and N2H+ are significantly inhibited by vibrational excitation of N (X2Σ
(X2Σ ) at Ecm = 0.05–0.40 eV. However, for the formation of CH
) at Ecm = 0.05–0.40 eV. However, for the formation of CH in this Ecm region, the σv+ values for v+ = 0, 1, and 2 are found to be nearly identical after taking into account the experimental uncertainties. Assuming that the formation of CH
in this Ecm region, the σv+ values for v+ = 0, 1, and 2 are found to be nearly identical after taking into account the experimental uncertainties. Assuming that the formation of CH and CH
and CH at low Ecm values is mediated by the dissociative charge transfer mechanism as suggested by previous studies, the formation of excited CH
at low Ecm values is mediated by the dissociative charge transfer mechanism as suggested by previous studies, the formation of excited CH * by charge transfer collisions of N
* by charge transfer collisions of N (X2Σ
(X2Σ ) + CH4 should reflect the vibrational energy resonance of N
) + CH4 should reflect the vibrational energy resonance of N (X2Σ
(X2Σ ) and CH
) and CH . The subsequent dissociation of excited CH
. The subsequent dissociation of excited CH * to form CH
* to form CH + H is likely to proceed via a loose transition state, which mostly involves stretching an H···CH
+ H is likely to proceed via a loose transition state, which mostly involves stretching an H···CH bond and is expected to have no potential energy barrier. Thus, the charge transfer mechanism can partially account for the observed vibrational dependences for σv+(CH
bond and is expected to have no potential energy barrier. Thus, the charge transfer mechanism can partially account for the observed vibrational dependences for σv+(CH ). In the case of the formation of CH
). In the case of the formation of CH + H2 from CH
+ H2 from CH *, the H2 elimination is likely involved in a tight transition state associated with a potential energy barrier, which could wash out the vibrational energy effect for reaction (3).
*, the H2 elimination is likely involved in a tight transition state associated with a potential energy barrier, which could wash out the vibrational energy effect for reaction (3).
In the region of Ecm = 0.50–1.00 eV, no vibrational effects are discernible for reactions (2)–(4), after taking into account the experimental uncertainties of about 10%. When Ecm is increased from 2.00 to 10.00 eV, a clear vibrational inhibition effect can be observed with σ0 > σ1 > σ2 for all three product channels, revealing the rich dynamics for this reaction system resulting from the nonadiabatic coupling involving the kinetic and vibrational motions of the N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 reaction system. In order to understand the experimental observations, rigorous dynamical calculations based on the accurate potential surfaces of the N
; v+ = 0–2) + CH4 reaction system. In order to understand the experimental observations, rigorous dynamical calculations based on the accurate potential surfaces of the N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 reaction system are needed.
; v+ = 0–2) + CH4 reaction system are needed.
To further illustrate the vibrational excitation on the overall reactivity of the N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 collisions, we have taken the sum of the absolute integral cross sections for all product channels σv+(CH
; v+ = 0–2) + CH4 collisions, we have taken the sum of the absolute integral cross sections for all product channels σv+(CH + CH
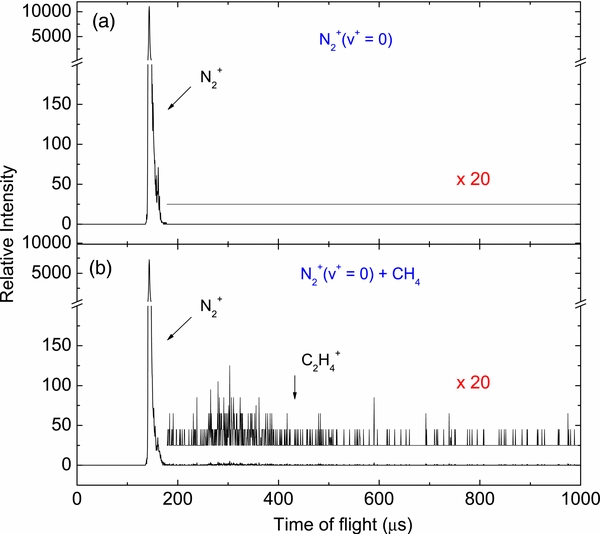
+ CH + N2H+), v+ = 0, 1, and 2, and have plotted them as a function of Ecm in Figure 5. These plots show similar trends on both kinetic and vibrational energy dependences for individual reaction channels shown in Figures 4(a)–(c). Although all previous cross sections and thermal rate constants (kT) for the N
+ N2H+), v+ = 0, 1, and 2, and have plotted them as a function of Ecm in Figure 5. These plots show similar trends on both kinetic and vibrational energy dependences for individual reaction channels shown in Figures 4(a)–(c). Although all previous cross sections and thermal rate constants (kT) for the N (X2Σ
(X2Σ ) + CH4 reaction available in the literature were concerned only with N
) + CH4 reaction available in the literature were concerned only with N (X2Σ
(X2Σ ) in the v+ = 0 ground state, we have included these results in Figure 4 for comparison with the present study. Here, the kT values were converted into absolute integral cross section (σ0) by using the relation σ0 = kT(μ/2Ecm)1/2, where μ is the reduced mass of N
) in the v+ = 0 ground state, we have included these results in Figure 4 for comparison with the present study. Here, the kT values were converted into absolute integral cross section (σ0) by using the relation σ0 = kT(μ/2Ecm)1/2, where μ is the reduced mass of N and CH4. The predictions based on the LGS model (Stevenson & Schissler 1958) are also depicted in Figure 5. The significantly smaller LGS predictions for the integral cross sections have been noted in previous studies (Nicolas et al. 2003), indicating that for the N
and CH4. The predictions based on the LGS model (Stevenson & Schissler 1958) are also depicted in Figure 5. The significantly smaller LGS predictions for the integral cross sections have been noted in previous studies (Nicolas et al. 2003), indicating that for the N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 reaction system, the interaction between N
; v+ = 0–2) + CH4 reaction system, the interaction between N and CH4 cannot be adequately described by the LGS model which only assumes the charge-induced dipole interaction. As shown in Figure 5, while the profile for the σv+(CH
and CH4 cannot be adequately described by the LGS model which only assumes the charge-induced dipole interaction. As shown in Figure 5, while the profile for the σv+(CH + CH
+ CH + N2H+) curve of Figure 5 obtained in the present study is in agreement with that reported by Nicolas and co-workers, the absolute cross-sectional values of this experiment are about 70% larger than that reported (Nicolas et al. 2003). We note that the absolute cross sections reported by Nicolas et al. were determined by calibrating their N
+ N2H+) curve of Figure 5 obtained in the present study is in agreement with that reported by Nicolas and co-workers, the absolute cross-sectional values of this experiment are about 70% larger than that reported (Nicolas et al. 2003). We note that the absolute cross sections reported by Nicolas et al. were determined by calibrating their N (X2Σ
(X2Σ ; v+ = 0) + Ar charge transfer cross sections with those measured by Schultz & Armentrout (1991). Considering that the uncertainties for individual absolute integral cross-sectional measurements by using the guided ion beam technique are ≈30%, the accumulated uncertainty for the absolute integral cross sections thus obtained by Nicolas et al. (2003) could be more than 30%. In our cross-sectional measurements, the ion lenses were carefully tuned to maximize the product ion transmission such that the sum of the product ion signals is close to the attenuation of the reactant H2O+ ion beam signal.
; v+ = 0) + Ar charge transfer cross sections with those measured by Schultz & Armentrout (1991). Considering that the uncertainties for individual absolute integral cross-sectional measurements by using the guided ion beam technique are ≈30%, the accumulated uncertainty for the absolute integral cross sections thus obtained by Nicolas et al. (2003) could be more than 30%. In our cross-sectional measurements, the ion lenses were carefully tuned to maximize the product ion transmission such that the sum of the product ion signals is close to the attenuation of the reactant H2O+ ion beam signal.
Figure 5. Sum of absolute integral cross sections for the formation of CH , CH
, CH , and N2H+ [σv+(CH
, and N2H+ [σv+(CH + CH
+ CH + N2H+) = σv+(CH
+ N2H+) = σv+(CH ) + σv+(CH
) + σv+(CH )] + [σv+(N2H+)] from the vibrationally state-selected reaction of N
)] + [σv+(N2H+)] from the vibrationally state-selected reaction of N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 obtained in the Ecm range of 0.05–10.00 eV. The standard deviations (≈10%) are determined by the reproducibility of independent measurements. Previous studies on absolute cross sections (Nicolas et al. 2003), thermal rate constants (kT) (Anicich et al. 2004; McEwan et al. 1998; Smith et al. 1978; Tichý et al. 1979), and the predictions based on the LGS model (Stevenson & Schissler 1958) for the N
; v+ = 0–2) + CH4 obtained in the Ecm range of 0.05–10.00 eV. The standard deviations (≈10%) are determined by the reproducibility of independent measurements. Previous studies on absolute cross sections (Nicolas et al. 2003), thermal rate constants (kT) (Anicich et al. 2004; McEwan et al. 1998; Smith et al. 1978; Tichý et al. 1979), and the predictions based on the LGS model (Stevenson & Schissler 1958) for the N (X2Σ
(X2Σ , v+ = 0) + CH4 reaction were included in the figure for comparison with the present study.
, v+ = 0) + CH4 reaction were included in the figure for comparison with the present study.
Download figure:
Standard image High-resolution imageBranching ratios for the three reaction channels (reactions (2)–(4), [σv+(CH ):σv+(CH
):σv+(CH ):σv+(N2H+)]/σv+(CH
):σv+(N2H+)]/σv+(CH + CH
+ CH + N2H+) for v+ = 0, 1, and 2, determined in the Ecm range of 0.05–10.00 eV are summarized in Table 1. As shown in the table, after taking into account the experimental uncertainties, the branching ratios for the three channels are found to be independent of Ecm and the v+ state of reactant N
+ N2H+) for v+ = 0, 1, and 2, determined in the Ecm range of 0.05–10.00 eV are summarized in Table 1. As shown in the table, after taking into account the experimental uncertainties, the branching ratios for the three channels are found to be independent of Ecm and the v+ state of reactant N (X2Σ
(X2Σ ) for v+ = 0–2 and Ecm = 0.05–9.00 eV. The branching ratios for v+ = 0 determined at Ecm = 0.05 eV are [σ0(CH
) for v+ = 0–2 and Ecm = 0.05–9.00 eV. The branching ratios for v+ = 0 determined at Ecm = 0.05 eV are [σ0(CH ):σ0(CH
):σ0(CH ):σ0(N2H+)]/σv+(CH
):σ0(N2H+)]/σv+(CH + CH
+ CH + N2H+) = 0.68 ± 0.05:0.26 ± 0.04:0.06 ± 0.01, which are quite different with previous (Anicich et al. 2004; Gichuhi & Suits 2011; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979) measurements. These ratios remain nearly the same until Ecm is increased to 9.00 eV. When Ecm is further increased to 10.00 eV, a branching ratio of 0.58 ± 0.05:0.34 ± 0.04:0.08 ± 0.01 is observed, indicating that the relative intensity for product CH
+ N2H+) = 0.68 ± 0.05:0.26 ± 0.04:0.06 ± 0.01, which are quite different with previous (Anicich et al. 2004; Gichuhi & Suits 2011; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979) measurements. These ratios remain nearly the same until Ecm is increased to 9.00 eV. When Ecm is further increased to 10.00 eV, a branching ratio of 0.58 ± 0.05:0.34 ± 0.04:0.08 ± 0.01 is observed, indicating that the relative intensity for product CH decreases while those for both products CH
decreases while those for both products CH and N2H+ become more important as Ecm is increased from 9:00 to 10.00 eV. As pointed out above, the variation and Ecm dependences of the branching ratios [σv+(CH
and N2H+ become more important as Ecm is increased from 9:00 to 10.00 eV. As pointed out above, the variation and Ecm dependences of the branching ratios [σv+(CH ):σv+(CH
):σv+(CH ):σv+(N2H+)]/σv+(CH
):σv+(N2H+)]/σv+(CH + CH
+ CH + N2H+) for v+ = 1 and 2 are essentially the same as those presented above for v+ = 0.
+ N2H+) for v+ = 1 and 2 are essentially the same as those presented above for v+ = 0.
Table 1. Branching Ratios for the Formation of CH , CH
, CH , and N2H+ from the Vibrationally Selected Reaction N
, and N2H+ from the Vibrationally Selected Reaction N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 Determined in the Ecm Range 0.05–10.00 eVa,b
; v+ = 0–2) + CH4 Determined in the Ecm Range 0.05–10.00 eVa,b
| v+ = 0 | v+ = 1 | v+ = 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecm | CH |
CH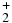 |
N2H+ | CH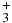 |
CH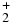 |
N2H+ | CH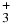 |
CH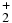 |
N2H+ |
| (eV) | |||||||||
| 0.05 | 0.68 | 0.26 | 0.06 | 0.66 | 0.29 | 0.05 | 0.68 | 0.26 | 0.06 |
| 0.10 | 0.70 | 0.24 | 0.06 | 0.68 | 0.27 | 0.05 | 0.69 | 0.26 | 0.05 |
| 0.20 | 0.68 | 0.26 | 0.06 | 0.67 | 0.28 | 0.04 | 0.69 | 0.27 | 0.04 |
| 0.30 | 0.68 | 0.27 | 0.05 | 0.65 | 0.30 | 0.05 | 0.66 | 0.30 | 0.04 |
| 0.40 | 0.67 | 0.28 | 0.05 | 0.65 | 0.32 | 0.03 | 0.67 | 0.29 | 0.04 |
| 0.50 | 0.65 | 0.30 | 0.05 | 0.63 | 0.33 | 0.04 | 0.66 | 0.30 | 0.03 |
| 0.60 | 0.66 | 0.30 | 0.04 | 0.64 | 0.33 | 0.03 | 0.67 | 0.30 | 0.03 |
| 0.70 | 0.65 | 0.31 | 0.04 | 0.64 | 0.33 | 0.03 | 0.67 | 0.30 | 0.03 |
| 0.80 | 0.65 | 0.31 | 0.04 | 0.64 | 0.33 | 0.03 | 0.65 | 0.32 | 0.03 |
| 0.90 | 0.63 | 0.33 | 0.04 | 0.63 | 0.33 | 0.04 | 0.65 | 0.31 | 0.04 |
| 1.00 | 0.64 | 0.32 | 0.04 | 0.66 | 0.31 | 0.03 | 0.65 | 0.32 | 0.03 |
| 2.00 | 0.64 | 0.32 | 0.04 | 0.63 | 0.34 | 0.03 | 0.65 | 0.32 | 0.03 |
| 3.00 | 0.63 | 0.33 | 0.04 | 0.63 | 0.34 | 0.03 | 0.65 | 0.31 | 0.04 |
| 4.00 | 0.63 | 0.32 | 0.05 | 0.63 | 0.33 | 0.04 | 0.65 | 0.31 | 0.04 |
| 5.00 | 0.66 | 0.29 | 0.05 | 0.64 | 0.33 | 0.03 | 0.62 | 0.33 | 0.05 |
| 6.00 | 0.64 | 0.30 | 0.06 | 0.63 | 0.32 | 0.05 | 0.62 | 0.34 | 0.04 |
| 7.00 | 0.64 | 0.29 | 0.07 | 0.62 | 0.33 | 0.05 | 0.63 | 0.31 | 0.06 |
| 8.00 | 0.63 | 0.30 | 0.07 | 0.66 | 0.28 | 0.06 | 0.63 | 0.31 | 0.06 |
| 9.00 | 0.59 | 0.33 | 0.08 | 0.55 | 0.37 | 0.08 | 0.53 | 0.40 | 0.07 |
| 10.00 | 0.58 | 0.34 | 0.08 | 0.56 | 0.35 | 0.09 | 0.57 | 0.33 | 0.10 |
Notes.
aThese branching ratios have been corrected for secondary reactions involving product CH and CH
and CH ions from the N
ions from the N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 reactions.
bThe standard deviations are measures of the reproducibility from independent measurements. The estimated branch-ratio uncertainties for CH
; v+ = 0–2) + CH4 reactions.
bThe standard deviations are measures of the reproducibility from independent measurements. The estimated branch-ratio uncertainties for CH , CH
, CH , and N2H+ branches are 5%, 4%, and 1%, respectively.
, and N2H+ branches are 5%, 4%, and 1%, respectively.
Download table as: ASCIITypeset image
The branching ratios for CH :CH
:CH and CH
and CH :CH
:CH :N2H+ in the temperature range from 10 to 300 K have been measured in seven previous experiments employing different experimental techniques (Anicich et al. 2004; Gichuhi & Suits 2011; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979). Among these experiments, all have reported the observation of CH
:N2H+ in the temperature range from 10 to 300 K have been measured in seven previous experiments employing different experimental techniques (Anicich et al. 2004; Gichuhi & Suits 2011; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979). Among these experiments, all have reported the observation of CH and CH
and CH as the major products, but only two have reported the detection of the N2H+ product channel. The branching ratios for CH
as the major products, but only two have reported the detection of the N2H+ product channel. The branching ratios for CH , CH
, CH , and N2H+ obtained in previous measurements (Anicich et al. 2004; Gichuhi & Suits 2011; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979) were found to be in the ranges of 80%–93%, 5%–20%, and 5%–15%, respectively, indicating that CH
, and N2H+ obtained in previous measurements (Anicich et al. 2004; Gichuhi & Suits 2011; McEwan et al. 1998; Nicolas et al. 2003; Randeniya & Smith 1991; Smith et al. 1978; Tichý et al. 1979) were found to be in the ranges of 80%–93%, 5%–20%, and 5%–15%, respectively, indicating that CH is the major primary product ion. After making the correction for secondary reactions of CH
is the major primary product ion. After making the correction for secondary reactions of CH and CH
and CH using the procedures outlined above, we have determined the branching ratios as [σ0(CH
using the procedures outlined above, we have determined the branching ratios as [σ0(CH ):σ0(CH
):σ0(CH ): σ0(N2H+)]/σv+(CH
): σ0(N2H+)]/σv+(CH + CH
+ CH + N2H+) = 0.68 ± 0.05:0.26 ± 0.04:0.06 ± 0.01 at Ecm = 0.05 eV. Since CH
+ N2H+) = 0.68 ± 0.05:0.26 ± 0.04:0.06 ± 0.01 at Ecm = 0.05 eV. Since CH is more reactive than CH
is more reactive than CH toward CH4, the net effect of making the correction for secondary reactions of CH
toward CH4, the net effect of making the correction for secondary reactions of CH and CH
and CH is enhancing the branching ratio for CH
is enhancing the branching ratio for CH and correspondingly suppressing that for CH
and correspondingly suppressing that for CH . If we only made the correction for reactions (5) and (6), i.e., for the formation of C2H
. If we only made the correction for reactions (5) and (6), i.e., for the formation of C2H , but not C2H
, but not C2H , as was done by Nicolas et al. (2003), we obtained the branching ratios as [σ0(CH
, as was done by Nicolas et al. (2003), we obtained the branching ratios as [σ0(CH ):σ0(CH
):σ0(CH ) and σ0(N2H+)]/σv+(CH
) and σ0(N2H+)]/σv+(CH + CH
+ CH + N2H+) = 0.75 ± 0.05:0.19 ± 0.04:0.06 ± 0.02 at Ecm = 0.05 eV, which are also included in Table 2. These partially corrected values are consistent with those reported by Nicolas et al. (2003) and Gichuhi & Suits (2011), and their co-workers, respectively.
+ N2H+) = 0.75 ± 0.05:0.19 ± 0.04:0.06 ± 0.02 at Ecm = 0.05 eV, which are also included in Table 2. These partially corrected values are consistent with those reported by Nicolas et al. (2003) and Gichuhi & Suits (2011), and their co-workers, respectively.
Table 2. Comparison of the Branch Ratios for the Formation of CH , CH
, CH , and N2H+ from the N
, and N2H+ from the N (X2Σ
(X2Σ ; v+ = 0) + CH4 reaction determined in the temperature (T) range 10–380 K
; v+ = 0) + CH4 reaction determined in the temperature (T) range 10–380 K
| Reference | CH |
CH |
N2H+ | T (K) |
|---|---|---|---|---|
| This worka | 0.65 ± 0.05 | 0.26 ± 0.04 | 0.06 ± 0.01 | |
| 380b | ||||
| (0.75 ± 0.05 | (0.19 ± 0.04) | (0.06 ± 0.02) | ||
| Smith et al. 1978 | 0.93 | 0.07 | 0 | 298 |
| Tichý et al. 1979 | 0.89 | 0.11 | 0 | 298 |
| Randeniya & Smith 1991 | 0.80 ± 0.10 | 0.20 ± 0.10 | 0 | 10 |
| McEwan et al. 1998 | 0.80 | 0.05 | 0.15 | 298 |
| Nicolas et al. 2003 | 0.86 | 0.09 | 0.05 | 298 |
| Anicich et al. 2004 | 0.88 | 0.12 | 0 | 298 |
| Gichuhi & Suits 2011 | 0.83 ± 0.02 | 0.17 ± 0.02 | 0 | 45 ± 5 |
Notes.
aThe branching ratios obtained at Ecm = 0.05 eV, which corresponds to T ≈ 380 K. These branching ratios have been corrected for secondary reactions of CH + CH4 and CH
+ CH4 and CH + CH4, leading to the production of C2H
+ CH4, leading to the production of C2H and C2H
and C2H . The values in parentheses were obtained without the correction for the production of C2H
. The values in parentheses were obtained without the correction for the production of C2H from the secondary reaction of CH
from the secondary reaction of CH + CH4. See the text.
bThe temperature of 380 K is converted from Ecm = 0.05 eV.
+ CH4. See the text.
bThe temperature of 380 K is converted from Ecm = 0.05 eV.
Download table as: ASCIITypeset image
4. CONCLUSIONS
Laser-based VUV–PFI-PI technique was employed to prepare reactant N ions in single-vibrational quantum states with high-purity, high-intensity, and high kinetic resolution, allowing the experimental investigations of the vibrationally selected ion–molecule reaction N
ions in single-vibrational quantum states with high-purity, high-intensity, and high kinetic resolution, allowing the experimental investigations of the vibrationally selected ion–molecule reaction N (X2Σ
(X2Σ ; v+ = 0–2) + CH4 in a wide Ecm range from 0.05 to 10.00 eV. Three product channels, which lead to the respective formations of CH
; v+ = 0–2) + CH4 in a wide Ecm range from 0.05 to 10.00 eV. Three product channels, which lead to the respective formations of CH , CH
, CH , and N2H+ ions, are observed. The σv+ values for these product channels are found to exhibit complicated vibrational dependences and vibrational inhibition for the N
, and N2H+ ions, are observed. The σv+ values for these product channels are found to exhibit complicated vibrational dependences and vibrational inhibition for the N + CH4 reaction is clearly evident. The σ0(CH
+ CH4 reaction is clearly evident. The σ0(CH + CH
+ CH + N2H+) values determined in the present study are larger than those reported in the guided ion beam study (Nicolas et al. 2003) and the predictions based on the LGS model (Stevenson & Schissler 1958). After correcting for the secondary reactions involving CH
+ N2H+) values determined in the present study are larger than those reported in the guided ion beam study (Nicolas et al. 2003) and the predictions based on the LGS model (Stevenson & Schissler 1958). After correcting for the secondary reactions involving CH and CH
and CH , the branching ratios for the three primary product channels [σv+(CH
, the branching ratios for the three primary product channels [σv+(CH ):σv+(CH
):σv+(CH ):σv+(N2H+)]/σv+(CH
):σv+(N2H+)]/σv+(CH + CH
+ CH + N2H+) determined here are different from those reported available in the literature. The lack of vibrational and kinetic energy dependences observed here are consistent with the result of previous study (Nicolas et al. 2003). Rigorous theoretical treatments are needed to fully understand all the phenomena observed. The branching ratios obtained in this study should be useful for the modeling of ion and neutral densities in the atmosphere of Titan. The cross-sectional data obtained in the present work should be useful for benchmarking theoretical predictions for ion–neutral collision dynamics.
+ N2H+) determined here are different from those reported available in the literature. The lack of vibrational and kinetic energy dependences observed here are consistent with the result of previous study (Nicolas et al. 2003). Rigorous theoretical treatments are needed to fully understand all the phenomena observed. The branching ratios obtained in this study should be useful for the modeling of ion and neutral densities in the atmosphere of Titan. The cross-sectional data obtained in the present work should be useful for benchmarking theoretical predictions for ion–neutral collision dynamics.
This work was supported by the NASA Planetary Atmospheres Program Grant No. 07-PATM07-0012. The construction of the DQDO apparatus used in this study was financed by the U.S. Department of Energy–Basic Energy Sciences (DE-FG02-02ER15306).