ABSTRACT
We have studied the reactions of polycyclic aromatic hydrocarbon cations and their nitrogen-containing analogs with H atoms. Reaction rate constants are measured at 300 K using a flowing afterglow-selected ion flow tube. We have implemented the laser induced acoustic desorption technique to allow the study of large, non-volatile species in the gas phase. The extension of this work from previous studies shows that the reactivity of polycyclic aromatic hydrocarbon cations with H atoms reaches a constant value for large cations. There is a small difference in reactivity when comparing molecules of different size and geometry; however, no difference in reactivity was found when nitrogen was incorporated into the ring.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
Polycyclic aromatic hydrocarbon (PAH) cations have long been proposed as possible carriers of the diffuse interstellar absorption bands (Sarre 2006; Pathak & Sarre 2008; Snow & Bierbaum 2008) and the unidentified infrared emission bands (UIRs; Léger & Puget 1984; Allamandola et al. 1985, 1999). The UIR bands are ubiquitous throughout the interstellar medium (ISM), vary with different environmental conditions (Peeters et al. 2002; van Diedenhoven et al. 2004), and show features characteristic of aromatic species (Li & Draine 2012). Although the mid-IR spectra will most likely not lead to the identification of individual PAHs, the generation of the NASA Ames PAH IR spectral database (Bauschlicher 2010) has aided in identifying classes of PAHs. Included in this list are PAH neutrals, cations, and anions varying in size (Cami 2011). In addition, polycyclic aromatic nitrogen heterocycles (PANHs) have been attributed to features in the IR emission spectra (Hudgins et al. 2005). Given that PAH cations are part of the IR emission spectra, it should be expected that PANH cations exist in these regions as well (Mattioda et al. 2008).
The origin of PAHs in the universe is still not completely understood; however, it has been likened to the PAH formation in the incomplete combustion of flames (Cherchneff et al. 1992). Several mechanisms of synthesis have been proposed for both radical–neutral and ion–neutral reactions for the formation of benzene and subsequently larger aromatic systems (Frenklach & Feigelson 1989; Cherchneff et al. 1992; Woods 2003; Cherchneff 2011; Soliman et al. 2012b; Parker et al. 2012). In addition, several computational studies have revealed mechanisms for incorporating nitrogen into an aromatic ring system (Ricca et al. 2001; Landera & Mebel 2010; Soliman et al. 2012a).
Whether the PA(N)H cations are formed from a direct reaction mechanism or by ionization of PA(N)H neutral molecules, these species will likely exist in the highest abundance in the diffuse regions of the ISM (Ruiterkamp et al. 2005). Here, atomic hydrogen dominates the chemical landscape and the hydrogenation and charge state of the molecules is determined by the size of the PA(N)H, radiation flux, and PA(N)H chemistry (Le Page et al. 2001). Alternatively, neutral PAHs and H2 are dominant in denser regions of the ISM. The study by Le Page et al. (2003) of the charge and hydrogenation state of PAHs in the diffuse medium concluded that PAHs with fewer than 30 carbons are either destroyed or severely dehydrogenated, whereas large PAHs (greater than 30 carbons) display normal hydrogenation in competition with the protonated form. Here, we have extended the studies previously carried out in our laboratory to include reactions of H atoms with additional PAH cations as well as with PANH cations. The structures of the species investigated are shown in Figure 1.
Figure 1. Names, structures, and numbering of the distinct positions of the PA(N)Hs included in this study.
Download figure:
Standard image High-resolution image2. METHODS
2.1. Experimental Methods
The experiments are carried out at 300 K using the flowing afterglow-selected ion flow tube (FA-SIFT) apparatus at the University of Colorado, Boulder (Figure 2). Details of the instrument and methods have been presented elsewhere (Bierbaum 2003; Snow & Bierbaum 2008). Here, PA(N)H neutral molecules that are sufficiently volatile are introduced into the gas phase through their vapor pressure at room temperature or with gentle heating when needed. The introduction of tetracene into the gas phase is achieved using the laser induced acoustic desorption (LIAD) technique, described below. The PA(N)H parent cations are generated by Penning ionization utilizing Ar metastables or chemical ionization with He+ (Diebold et al. 1980; Anderson et al. 1983; Le Page et al. 1999a). The reactant ions are then sampled, mass-selected using a quadrupole mass filter, injected into the reaction flow tube, and collisionally cooled by a helium carrier gas (0.30–0.45 Torr) prior to addition of neutral reactants. Atomic hydrogen is introduced into the reaction flow tube at a fixed reaction distance. Hydrogen atoms are generated by thermal dissociation of H2 gas on a hot tungsten filament. The reactant ion signal is monitored as a function of filament voltage using a quadrupole mass filter coupled to an electron multiplier. The filament voltage is directly correlated to the concentration of atomic hydrogen (Trainor et al. 1973), which is determined using the calibration reaction:
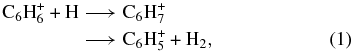
with a known reaction rate constant of 2.2 × 10−10 cm3 s−1 (Scott et al. 1997). Alternatively, measured concentrations of stable neutral reactant molecules are added to the reaction flow tube through a manifold of inlets. The reactant ion signal and kinetics are monitored as a function of reaction distance. Rate constants or upper limits in reactivity are derived using pseudo-first order kinetics (Bierbaum 2003).
Figure 2. FA-SIFT apparatus. Ions are generated in the ion production region using Penning ionization with argon metastables or chemical ionization with He+. Large non-volatile organics are introduced into the flow tube using the LIAD technique. Generated ions are then mass-selected by a quadrupole mass filter, and injected into the reaction flow tube, where H atoms are introduced at a fixed inlet. Ion intensities are measured as a function of H atom concentration using a quadrupole mass filter coupled to an electron multiplier. The H atom concentration is determined by a calibration reaction. Alternatively, stable neutrals are introduced through a manifold of inlets. The subsequent decrease in reactant-ion signal is monitored as a function of reaction distance.
Download figure:
Standard image High-resolution imageLarge carbonaceous species such as PA(N)Hs are difficult to study in the gas phase due to their extremely low vapor pressure and high melting point. Here, we utilize the LIAD technique which is an effective approach for introducing large, non-volatile species into the gas phase. This method has been well characterized previously for many different mass spectrometric applications (Shea et al. 2006, 2007a; Zinovev et al. 2007; Bald 2007; Nyadong et al. 2012). Although coronene cation has been studied in our FA-SIFT instrument previously, this required extreme temperatures and large quantities of solid coronene. The LIAD technique requires no heating and much less solid sample to attain similar ion intensities. Consequently, we have adopted this setup to our FA-SIFT to generate sufficient concentrations of tetracene cation in the gas-phase. First, a known amount of analyte is dissolved in benzene and deposited on a thin Ti foil (12.7 μm, Alfa Aesar). Multiple application techniques were explored including dipping the foil, adding the solution to the foil drop wise and allowing it to dry with and without heat, spin-coating, and airbrushing. Using an airbrush (Badger 200nh), we were able to apply a uniform coating of the analyte to a 3.1 cm diameter foil. Assuming all the dissolved solid is distributed evenly on the foil, a maximum density of ∼0.5 mg cm−2 is achieved. The airbrush technique has the advantage of efficient solute evaporation, which eliminates the aggregation effects seen in the other three techniques. After the analyte is applied to the foil, it is mounted on a probe where it is held in place between two rings. The probe is inserted into the ion production flow tube so that the foil is held in the center and desorption is perpendicular to the He flow. An unfocused, pulsed laser beam (Continuum Minilite II, Nd:YAG, 532 nm, 3 mm beam diameter, 15 Hz, 25 mJ pulse−1) impinges on the uncoated side of the foil, desorbing the intact, neutral analyte into the gas phase (Shea et al. 2007b). The laser beam is scanned to produce a continuous source of neutral analyte. Different scanning schemes were analyzed including random spot selection, spirals, vertical and horizontal methods. Starting at the upstream end of the foil, scanning vertically and subsequently moving downstream to obtain a new desorption area provides the most uniform ion intensities. The scanning procedure is achieved by a motorized mirror mount (Thorlabs KS1-Z8). A scan rate of 0.04 mm s−1 was found to give sufficient ion intensity while allowing maximum time per foil. This scan rate results in a desorption area of ∼8 ×10−3 mm2 per shot and a foil duration of 45 minutes. Argon metastables or He+ are generated upstream from the sample probe and the neutral analyte is subsequently ionized upon desorption. Ion intensities are directly correlated with laser power, which is consistent with previous work (Shea et al. 2007a; Zinovev et al. 2007).
2.2. Computational Methods
We have carried out density functional theory calculations to support experimental studies. We computed structures, geometries, and energies for reactants, intermediates, and products involved in the reactions using Gaussian 09 (Frisch et al. 2009) at the B3LYP/6-311G(d, p) level of theory (Lee et al. 1988; Becke 1993). This method and basis set have been shown to accurately determine energies of PAHs (Holm et al. 2011). Similar to the work of Holm et al. (2011), we have found only small differences (<0.5 kcal mol−1) in the computed reaction energies when comparing the basis sets 6-311G(d, p) and 6-311++G(2d, p). Therefore, reaction enthalpies are determined at 298 K using theoretically calculated geometries and energies with the 6-311G(d, p) basis set.
3. RESULTS AND DISCUSSION
The reactions of PA(N)H cations with H atom are summarized in Table 1; values include effective two-body reaction rate constants, collision rate constants, reaction efficiencies, and enthalpies of reaction. There is a total absolute error of 50% associated with the reported rate constants for reactions with H atom due to the experimental method. We report reaction efficiencies as kexp/kcol, where kcol is the collision rate constant determined by Langevin theory (Gioumousis & Stevenson 1958). Experimental enthalpies of reaction, ΔHexp, are determined using Hess's law with ionization energies, bond energies, proton affinities, and heats of formation taken from Linstrom & Mallard (2013) and from the 89th edition of the CRC Handbook of Chemistry and Physics (Lide 2008). In addition, we determine enthalpies of reaction at 298 K using density functional theory calculations. No measurable rate constants were obtained for reactions with H2, CO, NH3, H2O, and CO2. Therefore, we report a conservative upper limit rate constant of k ⩽ 1 × 10−12 cm3 s−1. All reactions with H atom proceed through association via a third body; however, in the ISM, radiative stabilization will likely preserve these association species. The reaction conditions of the experimental apparatus are not representative of interstellar temperatures and pressures (Snow & Bierbaum 2008); however, this systematic study combined with previous findings (Le Page et al. 1999a, 1999b; Betts et al. 2006) gives valuable insight into the reactivity of PAH and PANH cations. Our measured effective two-body rate constants can be used to compute a lower limit to the radiative association rate constant (Herbst et al. 1989). Although all reactions proceed via the same mechanism, two trends can be seen: (1) there are subtle differences in reactivity for PA(N)H cations of different size and geometry, and (2) there is no measurable difference in reactivity between PAH and PANH cations with H atom.
Table 1. PA(N)H Cation/H Atom Association Reactions
| M+ | kexpa | Efficiencyb | −ΔHexpc | Association at N | Association at C |
|---|---|---|---|---|---|
| −ΔHtheord | −ΔHtheord,e | ||||
| (10−9 cm3 s−1) | (kcal mol−1) | (kcal mol−1) | (kcal mol−1) | ||
| Naphthalene | 0.19f | 0.10 | 66.1 | ... | 64.5 |
| Quinoline | 0.19 | 0.10 | 113.2 | 109.7 | 72.0 |
| Isoquinoline | 0.18 | 0.095 | 110.6 | 108.4 | 67.0 |
| Anthracene | 0.13 | 0.068 | 67.7 | ... | 63.2 |
| Acridine | 0.14 | 0.074 | 98.8 | 97.5 | 61.9 |
| Phenanthrene | 0.17 | 0.090 | 65.7 | ... | 61.6 |
| Benzo[h]quinoline | 0.17 | 0.090 | ... | 96.7 | 65.1 |
| Phenanthridine | 0.17 | 0.090 | ... | 104.6 | 68.6 |
| Pyrene | 0.14g | 0.074 | 65.4 | ... | 62.8 |
| Tetracene | 0.13 | 0.068 | 63.6 | ... | 59.9 |
| Coronene | 0.14h | 0.074 | 60.4 | ... | 57.8 |
Notes. No measurable rate constants were obtained for reactions with H2, CO, NH3, H2O, and CO2. aRate coefficients are measured at 0.30–0.45 Torr helium. Total error is estimated to be ±50%. bThe reaction efficiency is reported as kexp/kcol. The collisional rate constant, kcol, is determined by Langevin theory (Gioumousis & Stevenson 1958) and gives a value of 1.9 × 10−10 cm3 s−1 for all reactions of PA(N)H+ with H atom. cValues determined using Hess's law with experimental values for ionization energies, bond energies, proton affinities, and heats of formation taken from Linstrom & Mallard (2013). The values correspond to association at C for PAH cations and to association at N for PANH cations. dB3LYP/6-311G(d, p) theory level including zero-point energy correction and thermal energy correction at 298 K. eReported values are calculated for association at the most exothermic position (see the Appendix). fLe Page et al. (1999b). gLe Page et al. (1999a). hBetts et al. (2006).
Download table as: ASCIITypeset image
3.1. Size and Geometry Dependence
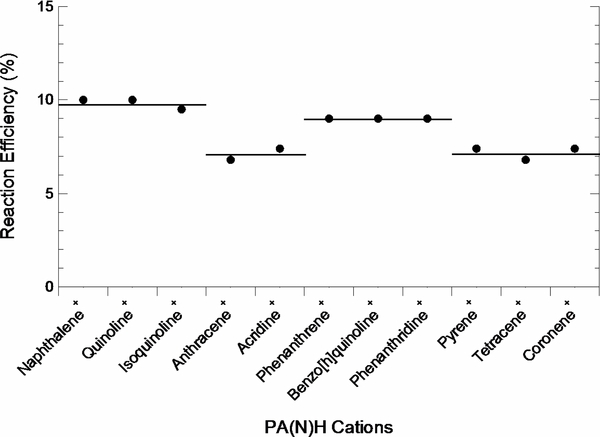
The reactions of PA(N)H cations with H atom all proceed through association to form a hydrogenated species. The association complex is stabilized in the FA-SIFT experiments by the He buffer gas. In the ISM, the density is too low for this to occur, and radiative association is the most likely mechanism for stabilizing the complex. Herbst & Le Page (1999) have shown that a PAH as small as naphthalene may have enough degrees of freedom to stabilize the association complex through radiative emission. In addition, the computed effective two-body rate constant remained constant over large temperature (50–300 K) and density (104–1016 cm−3) ranges. This phenomenon should be enhanced in the larger PA(N)Hs that are proposed to exist in the ISM due to the increased number of degrees of freedom. When comparing the PA(N)H cations included in this study, the reactivity of these species decreases with increasing size and quickly reaches an asymptote. This can be seen in Figure 3 when comparing the linear structures. The reaction efficiency reaches a constant value of ∼7%. The solid horizontal lines have been added to indicate that the encompassed data points are equivalent within experimental precision. It is also evident that the angular structure of phenanthrene, benzo[h]quinoline, and phenanthridine enhances their reactivity with H atom compared to the linear structures of anthracene and acridine. This may be explained by applying Clar's rule to the Kekulé structures; in this representation, three double bonds in one ring (a sextet) are replaced by a circle (Clar 1964). According to Clar's rule, the formula that best represents the molecule contains the greatest number of sextets (rings). In the case of phenanthrene, two different formulas can be drawn (Figure 4). Figure 4(a) is the best representative picture, with a ring at each end of the molecule and a fixed double bond in the center. This fixed double bond at the 5, 6 position reacts more like an olefin than an aromatic species in the neutral molecule, which may relate to the slight increase in reactivity in the parent cation (Clar 1964). However, this enhanced reactivity is relatively small (9% versus 7%).
Figure 3. Plot of the reaction efficiency for the reaction of PA(N)H cations with H atom. The solid horizontal lines have been added to indicate that the encompassed data points are equivalent within experimental precision. Reaction efficiencies are reported as kexp/kcol, where kcol is the collision rate constant determined by Langevin theory (Gioumousis & Stevenson 1958). Rate constants for naphthalene, pyrene and coronene were taken from Le Page et al. (1999b), Le Page et al. (1999a), and Betts et al. (2006), respectively.
Download figure:
Standard image High-resolution imageFigure 4. Two different Kekulé (left) and Clar (right) representations of phenanthrene. Clar's rule has been applied to the Kekulé structures to produce the Clar structures. In this representation, three double bonds in one ring are replaced by a circle. According to Clar's rule, the formula that best represents the molecule contains the greatest number of rings (4a; Clar 1964).
Download figure:
Standard image High-resolution image3.2. PAH versus PANH Cations
The reactivities of PAH and PANH cations with H atom are equivalent within experimental precision, when comparing PA(N)H cations of the same size and geometry (Figure 3). The exothermicities of the reaction for association at the nitrogen and the most exothermic carbon are listed in Table 1. For a complete list of calculated exothermicities see the Appendix. It has been suggested that the H atom associates at the most exothermic site for the reaction of PAH cations with H atom (Bauschlicher 1998). Although association at the nitrogen is almost twice as exothermic, the reactivity toward H atom is identical for PAH and PANH cations. We have explored the reaction coordinate for the interaction of quinoline cation with H atom and were unable to find any barriers for association of H atom at any site. However, there are barriers above the energy of the reactants for the transfer of the H atom between different sites. In addition, the triplet state association product of all PA(N)H+ cations with H atom is above the energy of the reactants. Given the lack of difference in reactivity, we suggest that the reaction of large PAH and PANH cations with H atom may be approximated by a single effective two-body rate constant, k = 1 × 10−10 cm3 s−1, which gives a lower limit to the radiative rate constant of k > 7 × 10−14 cm3 s−1 (Herbst et al. 1989).
4. CONCLUSIONS
We have continued the study of PAH cations previously carried out by our laboratory and extended the study to include nitrogen heterocycles. The LIAD technique was successfully adapted to an FA-SIFT to access large, non-volatile organic species. The LIAD technique can be used to study much larger PA(N)H ions and other non-volatile organic species; however, the pulsed nature of this technique coupled to the FA-SIFT makes studies of large species nontrivial. In addition, the results from this study indicate that a complete study of all possible PAH configurations may not be necessary. Several conclusions have become evident in this study:
- 1.PA(N)H cations are unreactive with H2, CO, NH3, H2O, and CO2 within our measurement capabilities. We place a conservative upper limit of k ⩽ 1 × 10−12 cm3 s−1.
- 2.PA(N)H cations show a slight difference in reactivity toward H atom for molecules of the same formula, but different geometries.
- 3.Reactions of H atom with PAH and PANH cations with the same structure and geometry exhibit no measurable difference in reaction efficiency.
- 4.The reaction efficiency of PA(N)H cations with H atom decreases slightly as the size of the cation increases but reaches a constant value of ∼7%.
- 5.
The modeled rate coefficient for the reaction of PAH cations with H atom used by Le Page et al. (2001) works well for small PAHs (<16 carbon atoms); however, it is evident from our results that larger PAHs deviate from this equation. For example, using the model suggested by Le Page et al. (2001), the rate constants for anthracene, tetracene, and coronene should be larger. In addition, there should be no difference in reactivity between anthracene and phenanthrene. Because it is likely that PA(N)H cations with 30–50 carbon atoms will have normal hydrogen coverage in the diffuse ISM, we suggest that a single rate constant is sufficient to describe the reactions of PA(N)H cations with H atom.
We acknowledge Ditte Thomsen for her helpful contributions and thank Professor Hilkka Kentämaa and her group for their insight and knowledge of implementing the LIAD technique. We express our sincere gratitude for financial support from NASA (NNX10AC78G) and NSF (CHE-1012321 and CHE-1300886). We also gratefully acknowledge support from the Extreme Science and Engineering Discovery Environment (XSEDE) computer resources provided by NSF.
APPENDIX:
The reaction thermodynamics for H atom association at each available position was also investigated. Listed in Table 2 are the PA(N)H cations, association products, experimentally determined energies where available, and theoretically calculated energies at 0 K and 298 K. PAH molecules are numbered according to IUPAC rules so that the maximum number of rings lie in a horizontal row and as many rings as possible are above and to the right of the horizontal row (Harvey 1991). Numbering starts with the most counter-clockwise carbon and commences in a clockwise manner counting only the exterior carbons. PANH molecules are oriented such that the nitrogen is placed in the lowest numbered position according to the rules above. Numbering starts at the nitrogen and proceeds clockwise. Figure 1 shows the PA(N)Hs investigated in this study and the distinct positions for association are numbered.
Table 2. Comparison of Reaction Exothermicities: PA(N)H Cations + H Atom
| M+ | Product | −ΔHexpa | −ΔHtheor(0K)b | −ΔHtheor(298K)b,c |
|---|---|---|---|---|
| (kcal mol−1) | (kcal mol−1) | (kcal mol−1) | ||
| Naphthalene+ | 1-hydronaphthalene+ | 66.1 | 63.2 | 64.5 |
| 2-hydronaphthalene+ | 60.3 | 61.6 | ||
| Quinoline+ | 1-hydroquinoline+ | 113.2 | 108.0 | 109.7 |
| 2-hydroquinoline+ | 56.8 | 58.2 | ||
| 3-hydroquinoline+ | 65.5 | 66.9 | ||
| 4-hydroquinoline+ | 56.5 | 57.8 | ||
| 5-hydroquinoline+ | 68.3 | 69.7 | ||
| 6-hydroquinoline+ | 65.9 | 67.3 | ||
| 7-hydroquinoline+ | 64.5 | 65.9 | ||
| 8-hydroquinoline+ | 70.6 | 72.0 | ||
| Isoquinoline+ | 1-hydroisoquinoline+ | 110.6 | 106.7 | 108.4 |
| 2-hydroisoquinoline+ | 60.9 | 62.3 | ||
| 3-hydroisoquinoline+ | 65.7 | 67.0 | ||
| 4-hydroisoquinoline+ | 65.6 | 67.0 | ||
| 5-hydroisoquinoline+ | 56.4 | 57.8 | ||
| 6-hydroisoquinoline+ | 62.3 | 63.7 | ||
| 7-hydroisoquinoline+ | 61.1 | 62.5 | ||
| 8-hydroisoquinoline+ | 58.2 | 59.6 | ||
| Anthracene+ | 1-hydroanthracene+ | 53.2 | 54.4 | |
| 2-hydroanthracene+ | 67.7 | 50.0 | 51.2 | |
| 5-hydroanthracene+ | 62.0 | 63.2 | ||
| Acridine+ | 1-hydroacridine+ | 98.8 | 96.0 | 97.5 |
| 2-hydroacridine+ | 60.7 | 61.9 | ||
| 3-hydroacridine+ | 52.4 | 53.6 | ||
| 4-hydroacridine+ | 56.0 | 57.2 | ||
| 5-hydroacridine+ | 56.7 | 58.0 | ||
| 6-hydroacridine+ | 56.5 | 57.7 | ||
| Phenanthrene+ | 1-hydrophenanthrene+ | 59.2 | 60.5 | |
| 2-hydrophenanthrene+ | 59.8 | 61.0 | ||
| 3-hydrophenanthrene+ | 65.7 | 58.2 | 59.4 | |
| 4-hydrophenanthrene+ | 60.3 | 61.6 | ||
| 5-hydrophenanthrene+ | 60.2 | 61.5 | ||
| Benzo[h]quinoline+ | 1-hydrobenzo[h]quinoline+ | 95.1 | 96.7 | |
| 2-hydrobenzo[h]quinoline+ | 53.1 | 54.4 | ||
| 3-hydrobenzo[h]quinoline+ | 58.0 | 59.2 | ||
| 4-hydrobenzo[h]quinoline+ | 50.2 | 51.4 | ||
| 5-hydrobenzo[h]quinoline+ | 62.0 | 63.3 | ||
| 6-hydrobenzo[h]quinoline+ | 60.0 | 61.3 | ||
| 7-hydrobenzo[h]quinoline+ | 63.8 | 65.1 | ||
| 8-hydrobenzo[h]quinoline+ | 59.5 | 60.8 | ||
| 9-hydrobenzo[h]quinoline+ | 62.8 | 64.1 | ||
| 10-hydrobenzo[h]quinoline+ | 61.9 | 63.2 | ||
| Phenanthridine+ | 1-hydrophenanthridine+ | 102.9 | 104.6 | |
| 2-hydrophenanthridine+ | 55.4 | 56.8 | ||
| 3-hydrophenanthridine+ | 59.0 | 60.4 | ||
| 4-hydrophenanthridine+ | 62.2 | 63.6 | ||
| 5-hydrophenanthridine+ | 57.1 | 58.5 | ||
| 6-hydrophenanthridine+ | 63.1 | 64.5 | ||
| 7-hydrophenanthridine+ | 65.4 | 66.8 | ||
| 8-hydrophenanthridine+ | 65.0 | 66.4 | ||
| 9-hydrophenanthridine+ | 64.1 | 65.6 | ||
| 10-hydrophenanthridine+ | 67.2 | 68.6 | ||
| Pyrene+ | 1-hydropyrene+ | 61.6 | 62.8 | |
| 2-hydropyrene+ | 65.4 | 47.1 | 48.3 | |
| 4-hydropyrene+ | 51.0 | 52.2 | ||
| Tetracene+ | 1-hydrotetracene+ | 46.6 | 47.8 | |
| 2-hydrotetracene+ | 63.6 | 43.7 | 44.9 | |
| 5-hydrotetracene+ | 58.7 | 59.9 | ||
| Coronene+ | 1-hydrocoronene+ | 60.4 | 56.3 | 57.8 |
Notes. aValues determined using Hess's law with experimental values for ionization energies, bond energies, proton affinities, and heats of formation taken from Linstrom & Mallard (2013). bB3LYP/6-311G(d, p) theory level including zero-point energy correction. cIncluding thermal energy correction at 298 K.
Download table as: ASCIITypeset image