Abstract
The study aims to investigate the effect of the addition of nanotubes of halloysite on the augmentation of chains observed in an aqueous magnetic fluid consisting of co-precipitated magnetite particles stabilized with lauric acid. Three samples of the mixture containing 0.5%, 1% and 2% of halloysite nanotubes (HNTs) and a pure magnetic fluid are used for this study. A room temperature magnetization study shows that for 0.5% and 1% of HNT, the magnetization of the mixture significantly increases, while for the higher concentration (2%) it decreases. Such concentration dependent behaviour on the addition of a nonmagnetic system to a magnetic fluid has not previously been observed. The increase in the magnetization is attributed to smaller sized (<5–6 nm) magnetite attached to the HNT, forming a magnetite–HNT composite. Additionally, field-induced chaining is augmented by the addition of HNT in the magnetic fluid. The augmentation of chain formation is confirmed by optical microscopy, field-induced transmission changes and field-dependent diffraction effects. The augmentation will be useful in enhancing other properties of the composite, such as the viscosity and thermal conductivity of nanofluids.
Export citation and abstract BibTeX RIS
1. Introduction
In the last two decades new or modified synthesis routes have been proposed for filling carbon nanotubes (CNTs) with nonmagnetic and magnetic nanoparticles—forming a nanoparticle–CNT composite—to prepare nanofluids [1–6]. In their pioneering work, Ajayan et al [1] successfully developed the oxidative cleavage of CNT end caps and subsequently filled them with nonmagnetic liquid metal. Korneva et al [4] have demonstrated the viability to use a capillary suction method for filling CNTs with magnetic fluid; the method was subsequently modified by others [5]. In all these works, the magnetite–CNT composite forms only when non-polar magnetic fluid is used, whereas in polar-magnetic fluid it fails due to the hydrophobic surface of the CNT [6]. Therefore, post-synthesis treated hydrophilic CNTs (e.g. etching) are used to prepare CNT-based polar nanofluids. Conversely, halloysite nanotubes (HNTs)—made using a naturally occurring mineral with surface hydrophilicity—have received less attention from the research community. The HNT has unique properties such as a hydrated surface (7–10 Å), a negative surface potential of SiO2 on the outer-walls and a positive potential of Al2O3 on the inner surface, providing multiple site binding. Therefore it does not require pre/post physical and/or chemical treatments.
In 1948, the organic complexes of halloysite were first studied systematically by Macewan [7], where they demonstrated that several highly polar organic liquids (e.g. simple alcohols, diols or alcohol ethers) form single-layer complexes with hydrated halloysite. This work was extended by Carr and Chih, where they had tested the complex formation of halloysite with polar–non-polar solvents on around 127 compounds [8]. They found that (i) compounds which form complexes with halloysite in general are polar and are usually either acids or bases, and (ii) complex formations are favoured by the presence of–OH and/or–NH2 functional groups. The applications of a halloysite composite cover a wide range, e.g. for the loading and control release of macromolecules, as an anticorrosion agent, as herbicides, insecticides, fungicides, and anti-microbial agents, in drugs and as food-additives [9].
Another material that has made a great impact on several fields of engineering and science is a magnetic fluid known as a ferrofluid. This fluid is categorized into non-polar fluids (e.g. hydrocarbons like kerosene, diester, etc.) and polar based magnetic fluids. In the former, a single layer of surfactant provides sufficient repulsive energy to overcome van der Waals and dipolar attractive forces; whereas, in the latter (e.g. a water-based magnetic fluid), charge determining ions and counter ions impart stability to the magnetic nanoparticles. In a water-based magnetic fluid when synthesized, the probability of stabilizing a single particle and particle clusters is finite. The net magnetic moment of these stabilized clusters is obviously higher than that of the single nanoparticle; therefore at a moderate magnetic field it attains a chain structure. Such a chain formation enhances the thermal conductivity and viscosity of the fluid, which may affect the efficiency of some devices such as seals or methods of drug delivery [10–13]. On the other hand, chain formation per se is a boon in the development of optical devices such as gratings and modulators [14, 15]. The formation of chains in magnetic colloids has been studied previously by several researchers [16–22]. Recently, Eloi et al investigated the field dependence optical transmission of tartrate-coated and polyaspartate-coated magnetite-based aqueous colloids. They showed that the magneto-transmissivity behaviour is mainly due to the rotation of linear chains, at the low-field range, whereas the analysis of the data provided the measurement of the average chain length. They also observed a minimum in transmissivity at a given critical magnetic field, whose origin is attributed to the onset of columns of chains built from isolated particle chains [23]. Magnetically induced changes in transmission for mixtures of nanomagnetic fluids (NMF) and other nonmagnetic particles have also been studied previously. Methods of the prevention as well as the promotion of such chain formation are useful for the development of devices based on NMF. The chain formation can be augmented in various ways, e.g. by the addition of micron sized nonmagnetic spheres (e.g. polystyrene [25]), nanosized nonmagnetic particles (e.g. latex [24]), or micron sized magnetic particles (magnetite [26, 27]).
In the present work, our main objective is to explore the possibility of augmentation of magnetic field induced chain formation in water-based magnetic fluids by the addition of HNTs. To our knowledge, this aspect has not been covered in earlier works. However, a report exists on the chemical deposition of cobalt nanoparticles on the surface of HNT [28]. Moreover, an overall improvement in the performance of HNT–nanoparticle composite nanofilms by the adsorption of organic macroions on surface-modified HNT has been described by several researchers [29, 30]. It has also been used as a substrate to fabricate a one-dimensional nanocomposite [31]. In all these works, researchers have used chemical deposition methods. In the present work, the HNT is mixed with an aqueous magnetic fluid to form HNT–magnetic fluid composites. The present work shows that for low concentrations of HNT (0.5% and 1%), the magnetization at 1 T increases, while on further increasing the HNT concentration, the magnetization value decreases. Magneto-optical study reveals that the addition of HNT to the fluid augments the field-induced chaining.
2. Experimental details
2.1. Sample preparation
An aqueous magnetite magnetic fluid stabilized with dodecanoic acid is prepared using the method of Khalafalla and Reimers [32]. Details of the synthesis and characterizations of the magnetic fluid are described in [33]. The structural analysis determined is as follows: crystal structure: fcc, space group: Fd3m, crystallite size: 8.9 ± 0.2 nm, lattice parameter: 0.8388 ± 0.0001 nm. The magnetic volume fraction (φm) of the fluid is 0.0713. The fluid is diluted to set φm = 0.014 and is coded as NMF.
Halloysite (Al2Si2O5(OH)4 · 2H2O) nanoclay (Sigma-Aldrich) has an average tube diameter and inner lumen diameter of 50 nm and 15 nm respectively. The length of the tube ranges from 0.5 to 2 µm. The bulk density of the nanoclay is 15.6 g ml−1 and a typical specific surface area = 65 m2 g−1; a pore volume of ∼1.25 ml g−1; a refractive index of 1.54; and a specific gravity of 2.53 g cm−3.
Three samples of HNT–magnetic composites are prepared by mixing 0.5%, 1% and 2% HNT powder (w/v) in aqueous magnetic fluid and labelled the mixtures as A2, B2 and C2, respectively.
2.2. Magnetization measurements
Magnetization measurement is carried out at 298 K using a vibrating sample magnetometer (Lakeshore model 7404) in the magnetic field range of +1 T to −1 T, in steps of 0.05 T.
2.3. Microscope study
An optical microscope (MAGNUIS MLXi) with a 40×, numerical aperture (NA) = 0.65 air objective attached to a digital camera (Sony DSC-W510) is used to record the dynamics of the magnetic-field-induced chain formations. The direction of the homogeneous magnetic field (0.05 T) is perpendicular to the sample.
2.4. Transmission measurements
The optical set up consists of an unpolarized He–Ne laser (Spectra-Physics) with a wavelength λ = 632.8 nm and a 2 mW output power. An external polarizer is used to set the axis of the electric vector
 of the light parallel to the direction of the magnetic field
of the light parallel to the direction of the magnetic field
 generated by an electromagnet. The magnet is oriented such that the direction of the field remains perpendicular (transverse) to that of the incident light. A constant current power supply is used to vary the field from 0 to 0.18 T. A glass sample cell of path length ∼1 mm is placed at the centre of the pole pieces of the electromagnet. Transmitted light from the cell is detected by a silicon diode having effective area ≈10 mm [2] and measured using a differential amplifier. A Hall probe is used to ascertain the uniformity of the field between the pole pieces.
generated by an electromagnet. The magnet is oriented such that the direction of the field remains perpendicular (transverse) to that of the incident light. A constant current power supply is used to vary the field from 0 to 0.18 T. A glass sample cell of path length ∼1 mm is placed at the centre of the pole pieces of the electromagnet. Transmitted light from the cell is detected by a silicon diode having effective area ≈10 mm [2] and measured using a differential amplifier. A Hall probe is used to ascertain the uniformity of the field between the pole pieces.
2.5. Time-dependent light scattering
Time-dependent light scattering intensity and diffraction patterns are recorded in the presence of a constant magnetic field.
3. Results and discussion
Figure 1(a) shows the variation of magnetization with the field of NMF, A2, B2 and C2 fluids recorded at 300 K. All the fluids exhibit a so-called extrinsic 'superparamagnetic' nature, i.e. zero coercivity or remanent magnetization. It should be recalled here that this superparamagnetic behaviour is quite distinct from that purely intrinsic superparamagnetism due to Néel rotation. This aspect is discussed by Rosensweig [34]. It is observed that upon the addition of HNT, a variation in saturation magnetization occurs. A superimposition of all the magnetization curves (M/MS) indicates that the addition of HNT has not changed the magnetic nature of the samples (inset figure 1(b)).
Figure 1. (a) Variation of magnetization with field recorded at 300 K for a nanomagnetic fluid (NMF), and magnetic fluids containing different concentrations of HNT (A2, B2 and C2). Inset: (b) superimposition of reduced magnetization curves of all the fluids, and (c) variation in the magnetization at H = 1 T for various concentrations of HNT.
Download figure:
Standard image High-resolution imageSince the magnetic volume fraction in all fluid is very low (<2%), it resembles a paramagnetic gas. Thus it can be analysed using Langevin's theory for paramagnetism incorporating size distribution. In a magnetic fluid, particle size distribution is found to obey a log-normal distribution. Accordingly, the magnetization of the fluid is given by

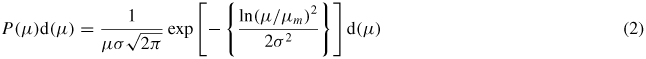
Here, the Langevin parameter α = μ H/kBT, μ is the magnetic moment of the particle, H is the applied magnetic field, kB is the Boltzmann constant, T is the absolute temperature, μm is the mean magnetic moment and σ is the log-normal moment distribution. In figure 1(b), the experimental data are compared with Langevin's theory (solid line). The parameters obtained from the fit are the mean magnetic moment, μm = 1.4 × 10−19 A m2, and the log-normal moment distribution, σ = 1.4. On the addition of HNT, μm and σ remain constant, but variation in the saturation magnetization is observed. Figure 1(c) shows the variation in the magnetization value obtained at H = 1 T. It is evident from figure 1(c) that, on the addition of 0.5% HNT (A2 fluid), the magnetization is enhanced by 23%, whereas for 1.0% HNT (B2 fluid), it is enhanced by 19%. On the other hand, the addition of 2% HNT (C2 fluid) reduces the magnetization by ∼11%.
A similar enhancement in magnetization upon the addition of polystyrene latex nonmagnetic particles in a ferrofluid has been observed by Islam et al [25]. They used a commercially available water-based ferrofluid with an average magnetic particle diameter ≈20 nm and latex spheres of diameters between 42 and 200 nm [25]. They also observed that the effective saturation magnetization of the ferrofluid particles increased by 7% as the volume fraction of the added 42 nm nonmagnetic beads increases from 0% to 20%. The reasons for this increase were not investigated. Later, only in a diluted ferrofluid, it was shown that the internal chain orientation correlations and the field-dependent chain lengthening result in a higher magnetization of the aggregated ferrofluid in comparison to the Langevin magnetization [26]. The authors have not considered inter-chain interaction and generalized the theory of particle association in a diluted magnetic fluid. It was also shown that the magnetization is weaker for a flexible chain than in the case of rigid rods.
In the present system, the magnetization data superimposes even after the addition of HNT. The agreement of the experimental data with Langevin's theory confirms that mean magnetic moment and moment distribution do not change on the addition of HNT. Considering Md = 485 kA m−1 (the bulk value of Fe3O4), the equivalent diameter of the magnetic particle remains within the range of 3–23 nm, with a mean particle diameter of 11.3 nm. It is known that the thermal energy of smaller-sized particles (<5–6 nm) is higher, and hence they do not contribute to the magnetization process. The evidence of enhancement in the magnetization can be attributed to the formation of a magnetite–Halloysite complex, which consists of smaller sized (<5 nm) magnetic particles. The magnetization process in the dispersion depends on the Langevin parameter α—the ratio of magnetic to thermal energy (μ H/kBT)—and as the thermal energy reduces, the effective magnetization increases. Halloysite exhibits a diamagnetic nature and also has shape anisotropy; hence on increasing the HNT concentration, the effective magnetization reduces. Detailed investigations are needed to understand the nature of interaction of HNT–magnetic nanoparticles. Since the main aim of the present work is to induce the augmentation of chain formation in a HNT–magnetic fluid composite, we shall concentrate on the further confirmation of augmentation. The following section discusses the magneto-optical transmission study.
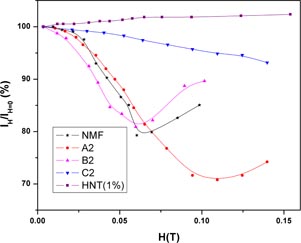
Figure 2 shows the field-induced normalized transmitted light intensity percentage (i.e.
 for
for
 configuration for NMF, A2, B2, C2 and HNT (1%). The field-induced transmitted intensities in all the three HNT–magnetic composites are different than the NMF one. In the NMF, the intensity decreases with increasing field, reaches a minima at H ≈ 0.065 T and then increases. In the A2 fluid, the intensity decreases rapidly with the field and the minima is now observed at HMax ≈ 0.1 T. However, for B2, the minima is observed at HMax ≈ 0.065 T but the magnitudes of the changes are comparatively larger than in the NMF. On the contrary, a very slow and almost linear decrement in intensity, and less magnitude, is observed in C2. In order to understand the magnetic-field-induced optical nature of HNTs, a 1% suspension in ammoniated water is prepared. The transmitted intensity of halloysite (1%) dispersion increases with the increasing magnetic field, which is due to the diamagnetic nature of the tube shaped HNT particles.
configuration for NMF, A2, B2, C2 and HNT (1%). The field-induced transmitted intensities in all the three HNT–magnetic composites are different than the NMF one. In the NMF, the intensity decreases with increasing field, reaches a minima at H ≈ 0.065 T and then increases. In the A2 fluid, the intensity decreases rapidly with the field and the minima is now observed at HMax ≈ 0.1 T. However, for B2, the minima is observed at HMax ≈ 0.065 T but the magnitudes of the changes are comparatively larger than in the NMF. On the contrary, a very slow and almost linear decrement in intensity, and less magnitude, is observed in C2. In order to understand the magnetic-field-induced optical nature of HNTs, a 1% suspension in ammoniated water is prepared. The transmitted intensity of halloysite (1%) dispersion increases with the increasing magnetic field, which is due to the diamagnetic nature of the tube shaped HNT particles.
Figure 2. Normalized transmitted light intensities as a function of magnetic field for the magnetic fluid NMF, the suspension of HNT in water and mixtures of NMF and HNT with different concentrations of HNT.
Download figure:
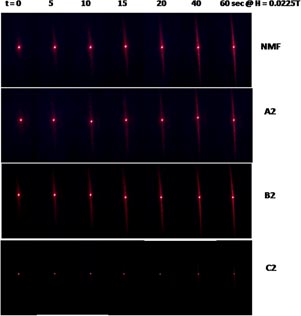
Standard image High-resolution imageTime-dependent chain formation and its microscopic confirmations have been recorded for NMF and all three HNT–magnetic composite fluids at H = 0.0225 T with different time intervals (figure 3). It is found that both the length and thickness of the chain, as well as agglomerates, increase with time. As discussed earlier, the formation of an HNT–magnetite composite has modified the magnetic properties. The length of such magnets (see figure 3: A2–C2) will be in the range of 0.5 to 2 µm, which results in larger chains compared to the NMF chains. It is found that both the length and thickness of the chain, as well as agglomerates, increase with time. It is noticed that in the C2 fluid, the number of chains per unit area is less than in the A2 and B2 fluids. There may exist some bare diamagnetic halloysite tubes (bluish in colour) that orient perpendicular to the field. Accordingly, fewer chains are observed in C2 (figure 3(c)). It is also known that field-induced chains result in a distinct diffraction line [35, 36]. This is also confirmed in the present case. Figure 4 shows the time-dependent diffraction pattern of NMF, A2, B2 and C2 fluid recorded at a constant magnetic field of 0.0225 T. In the NMF, A2 and B2 fluids, at this moderate magnetic field, as the chain formation progresses with time, the diffracted spot scatters into a line. It is to be noted that at t = 0 s, a small line along with the diffracted spot is observed, which may be due to presence of pre-clusters or agglomerates. The intensity of the diffracted line increases on the addition of 0.5% and 1% HNT in the A2 and B2 fluid respectively. The presence of the pre-clusters or agglomerates can also be seen in the respective microscopic images. The time-dependent diffracted spot observed in C2 is totally different to the other two systems. Similarly, distinct data is also observed in the magnetic-field-induced transmitted light intensity in this fluid. The diameter of the diffracted spot at t = 0 s is largely reduced with increasing time, and it remains almost same up to t = 20 s, and thereafter a very thin diffracted line along with the spot is observed.
Figure 3. Microscopic confirmation of chain formation and its augmentation observed at different time intervals, by the addition of HNT. The field strength was kept fixed at H = 0.0225 T.
Download figure:
Standard image High-resolution imageFigure 4. Magnetic field induced light diffraction recorded at different time intervals.
Download figure:
Standard image High-resolution imageAs stated above, in a diluted water base magnetic fluid (like an NMF), field-induced chain formation is usual. The present investigations reveal that the introduction of HNTs in such a fluid augments the chain formation. This is also confirmed from microscopic images. To explain the observed behaviour, two possibilities are considered: (i) geometrical shadow approximation, and (ii) chain interaction and a structural coarsening model.
Geometrical shadow approximation. In this, the transmission is considered to depend on the geometrical shadows of solid particles, especially when the size of the scatterer is larger than the wavelength of light [37]. Accordingly, in an absence of an external magnetic field, the particles are distributed at random, and their shadow obstructs the path of the light beam. Hence, light transmission will be low. When a field is applied, the particles are aligned and form chains along the direction of the field. This results in a net decrease in the shadowing effect and transmission increases. The number and thickness of the chains grow with the increase in the field, resulting in a decrement in transmission. At a critical field, field-induced chain formation reaches saturation. Consequently, the induced transmission changes also should reach saturation. However, in a fluid with a large dilution, there may be smaller agglomerates of spherical shapes as well as single nanoparticles. Moreover, these agglomerates grow in size with the increase in the field. At a field > Hmax, shadows of the agglomerates decrease the transmission resulting in an increase in intensity. This approximation is only applicable to explain the transmission changes in the system. In order to explain the enhancement of the magnetization observed after adding HNTs, we use the model first proposed by Halsey and Toor to describe structures induced by an externally applied electric (magnetic) field in electrorheological (magnetorheological (MR)) fluids [38–41].
Chain interaction and structural coarsening model. As stated above, in a magnetic colloid comprising of only nanomagnetic particles, chains and columns are formed upon increasing the applied magnetic field. The critical magnetic field (Hc) above which this columnar phase transition occurs can be estimated by equating the short-range direct interaction between particle chains and the fluctuation energy of a single particle chain within the column [42]. Our system is not a magnetic fluid in the true sense, but it is a mixture of HNTs on which magnetite spheres are attached and a surrounding medium containing the free nanomagnetic spheres. Thus, it is more similar to MR fluids. For this, the model proposed by Halsey and Toor for electrorheological fluids is better suited. According to this model, when a large field is applied, particles in the fluid form chains and columns along the direction of the field which extend from one end of the cell to the other. A cross-section of the chain will have only a single particle, while a column consists of a number of such chains. In the absence of an external magnetic field, the particles are governed by Brownian motion, and hence the net magnetization will be zero. In the presence of an external magnetic field, the magnetic moment (m) of an individual particle is given by

where a is the diameter of the nanoparticle, χ the effective susceptibility of the particle and H0 the magnitude of the external magnetic field. Pairs of particles with moments m interact via the anisotropic dipolar potential energy
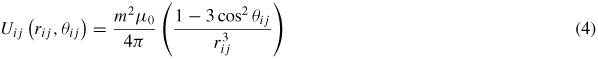
where, μ0 is the magnetic permeability of free space, rij the magnitude of the vector describing the distance between the centres of the ith and jth nanoparticles, and θij the angle between the vector rij and the external field vector. The relative strength of dipolar interactions and thermal effects can be characterized by the dimensionless parameter

Here, KB is the Boltzmann constant and T is the absolute temperature. For λ ≫ 1, the magnetic nanoparticles in the dispersion self-assemble into aligned structures. As the field increases, the moments of the particles start to align along the direction of the field resulting into dipolar chains, and they exhibit strong Landu–Peierls fluctuations. Halsey and Toor (HT) showed that these fluctuations lead to long-range coupling between dipolar chains, which results in an attractive interaction, with a power-law decay. This theoretical model was extended by considering time scales of interactions [38] and the local lateral field that can drive coarsening in the absence of thermal fluctuations [41].
According to the HT model, the longitudinal and transverse particle fluctuations of the wave vector k in a dipolar chain create local variations in the concentrations of dipoles. This, in turn, introduces fluctuations in the lateral field. The total interaction energy of two chains is the sum of the interaction energy between their fluctuations and the energy of deformation required for the fluctuation mode. Halsey and Toor assumed that in the first-order deformation, transverse fluctuations perpendicular to the plane defined by the chains do not contribute to the interaction energy. The deformation energy due to the fluctuation mode depends on longitudinal ( l) and transverse (
l) and transverse ( t) stiffness energies, which scale as m2/μ0a3.
t) stiffness energies, which scale as m2/μ0a3.
The total energy contributes to the partition function, with the result that the free energy for the chains as a function of ρ, given in equation (6), decays as a power law.

Here, ρ is the distance between two chains or columns. It is important to note that this calculated interaction is independent of the applied magnetic field. In fact, the interaction increases with the applied magnetic field, and its effect is cancelled out by a suppression of the fluctuations as the chains stiffen. The effect of the applied magnetic field strength was considered, and the modified HT model is given by Martin et al [41]:

This interaction energy can be either repulsive or attractive. This energy has a strong dependence on the magnetic field strength (H) and separation (ρ) between the chains. As the field increases, the structural transition of the system takes place at different critical fields. In the case of different critical fields, when ρ remains the same, the magnetic field, H, increases, resulting in an increase in the interaction energy per unit length, U. Two chains laterally coalesce when this energy becomes sufficiently high to overcome the potential barrier for the lateral aggregation. Due to lateral aggregation at the critical fields, the separation distance, ρ, between the columns now increases, which results in a decrease in U. This decreases the overall energy of the system.
As shown in figure 2, in the dispersion with only HNTs, the magnetic-field-dependent transmitted light intensity increases with the field, which is typically a diamagnetic behaviour. On the other hand, in a colloidal solution of only nanomagnetic particles (NMF), the transmitted intensity first decreases with the field, reaches a minimum and with further increase in the field, it increases. As shown in our previous work, this behaviour is due to the presence of a small number of large aggregates [43]. Hence, in a suspension containing both diamagnetic and ferrimagnetic particles, a contribution due to diamagnetic as well as ferrimagnetic particles (nanomagnetic and their aggregates) will describe the properties of the suspension. The former may lead to lateral (perpendicular to the applied field) interactions and the latter may lead to longitudinal (parallel) interactions. Thus, in A2, B2 and C2 fluids both types of interactions will occur, while in the case of pure NMF only longitudinal interaction will be dominant. Accordingly, as we increase the proportion of halloysite, the total interaction energy increases and this leads to an enhancement in the magnetization. The enhancement of magnetization observed in the samples A2 and B2 (figure 1) may be attributed to the increase in the interaction energy. The reduction in the magnetization observed for a 2% addition of halloysite can be explained as follows. If the concentration of the larger size diamagnetic particles is increased beyond a critical value then the effect of lateral interactions will be dominant and longitudinal interaction, due to smaller sized nanomagnetic particles, will be masked. Consequently, the net interaction energy for the sample C2 will be smaller than that for A2 and B2. As a result, the magnetization of the sample C2 is reduced. This is further corroborated by transmission study. For C2, the change in transmission intensity with field is very small, hence the contribution due to nanosized magnetic particles seems to be masked. As a result transmission changes are monotonically decreased with the field. In A2 and B2, the lateral interaction due to (0.5% and 1% respectively) halloysite will be added. In A2, a larger magnetic field is required to overcome the potential barrier than in B2. In B2, a larger amount of halloysite is present, hence the transverse interaction becomes stronger, and as a result the critical field at which chain separation starts is reduced to a low magnetic field compared to that in an NMF. Although the magnetization of B2 is higher than in the NMF, one expects similar behaviour as in A2, but the effect of the HNT concentration is clearly observed here. Similar behaviour is also reflected in the microscopic images (figure 3). Here the total number of chains per unit area for C2 decreases compared to that for A2 and B2 samples.
4. Conclusion
The present study shows that the addition of tube shaped halloysite nanoparticles leads to the augmentation of magnetic fluid chains in the aqueous nanomagnetic fluids. The augmentation is attributed to the increase in the magnetization of the mixture. The augmentation of chains is confirmed by three independent experimental investigations namely transmission changes, microscopic observations and time-dependent light diffraction. The effect of the addition of halloysite nanotubes in nanomagnetic fluids on the magnetization of the fluid is explained on the basis of the Halsey and Toor model. The findings will be useful for enhancement of viscosity and the thermal conductivity of magnetic fluids, which will be useful in design of several magneto-fluidics devices.
Acknowledgments
The work is supported by CHARUSAT research support grant and Department of Science and Technology project (DST-161-G).