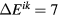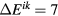Abstract
This work proposes a complete and consistent set of cross sections for electron collisions with carbon dioxide (CO2) molecules to be published in the IST-Lisbon database with LXCat. The set is validated from the comparison between swarm parameters calculated using a two-term Boltzmann solver and the available experimental data. The importance of superelastic collisions with CO2(0 1 0) molecules at low values of the reduced electric field is discussed. Due to significant uncertainties, there are ongoing debates regarding the deconvolution of cross sections that describe generic energy losses at specific energy thresholds into cross sections that describe individual processes. An important example of these uncertainties is with the dissociation of CO2, for which the total electron impact dissociation cross section has not yet been unambiguously identified. The available dissociation cross sections are evaluated and discussed, and a strategy to obtain electron-impact dissociation rate coefficients is suggested.
Export citation and abstract BibTeX RIS
1. Introduction
Carbon dioxide (CO2) is an important component of our atmosphere and a dominant constituent in the atmospheres of Venus and Mars. It has been extensively studied in the last few years due to its importance for dry reforming, space mission research and its effect on climate change [1–3]. The latter has recently put forward the idea of CO2 conversion as one of the major scientific and technological challenges of the modern world and has set goals for fundamental experimental research and plasma modelling [4–10]. In particular, an efficient storage of energy in chemical compounds produced from CO2 emissions would be extremely interesting from the environmental, economical and societal points of view. The main task to achieve this efficient storage of energy is to maximize the energy efficiency of CO2 dissociation. Low-temperature plasmas can promote CO2 dissociation by direct electron impact or by an indirect route passing through vibrational excitation [4–7]. However, the kinetic mechanisms leading to CO2 dissociation are not yet completely understood.
Electron collisions where molecules initiate all the processes in gas discharges, represent the starting point for plasma chemistry. A reliable quantification of a complete set of electron-impact cross sections is of immense significance for the study of the electron kinetics and the calculation of accurate electron energy distribution functions (EEDFs). In addition, it is desirable to identify the excited states and mechanisms corresponding to all the cross sections required to calculate the EEDFs; this allows us to gain a deeper insight into the elementary processes occurring in plasmas. Electron-impact dissociation of CO2 has been a topic of interest for many years. However, despite the numerous research addressing the question, the cross section for this process remains a missing piece of the puzzle [11–21]. In fact, there is no consensus on this matter and the lack of experimental data regarding the dissociation rate coefficient makes the validation of the electron-impact dissociation cross section of CO2 a very challenging subject.
To allow for the physical interpretation of experiments and to meet the demands of modelling, a comprehensive cross section set regarding plasma physics should be more than a simple collection of data. The electron momentum loss, the inelastic energy loss and electron number changing processes (e.g. ionization and attachment), as well as an identification of relevant superelastic gain processes, should be properly described. A set that provides the afore-stated descriptions is said to be complete. An EEDF can be calculated when a complete set of cross sections is used as an input to a Boltzmann solver or to Monte Carlo/particle-in-cell codes. The result depends on the working conditions and may deviate considerably from a Maxwellian. The transport parameters and collision rate coefficients can then be calculated from the first two moments of the Boltzmann equation. An equally important requirement a cross section set should fulfil is the ability to reproduce measured electron transport parameters and rate coefficients. A set that meets this requirement is said to be consistent. One of the strategies to determine a complete and consistent cross section set includes adjusting iteratively the magnitudes and/or shapes of an existing compilation of data (if possible within experimental uncertainty) to improve the agreement between calculated and measured swarm parameters. This 'swarm derivation' method is very useful and widely used in the low-temperature plasma community, but undermines the uniqueness of the cross section set and may complicate the identification of individual collision processes.
The aims of this paper are to propose a complete and consistent set of electron-neutral scattering cross sections for CO2 and to assess the available cross sections for dissociation. The set is swarm derived, based on the calculations from the two-term Boltzmann solver BOLSIG + [22], and will be shortly published on the IST-Lisbon database with LXCat. In addition, the existing dissociation cross sections are compared and evaluated, leading to a recommended procedure to calculate the rate coefficients of CO2 dissociation by electron impact.
The organization of this paper is as follows. The next section is devoted to the description of the complete and consistent set of electron-impact cross sections for CO2. The comparison of the two-term Boltzmann calculation of the swarm parameters with the existing measurements is presented and discussed in section 3, in which the importance of the superelastic collisions at low reduced electric fields ( Td, where E is the electric field and N is the gas density;
Td, where E is the electric field and N is the gas density;  Vm2) is also discussed. Section 4 evaluates and recommends cross sections for providing electron-impact rate coefficients for CO2 dissociation. Finally, the concluding remarks are summarized in section 5.
Vm2) is also discussed. Section 4 evaluates and recommends cross sections for providing electron-impact rate coefficients for CO2 dissociation. Finally, the concluding remarks are summarized in section 5.
2. The cross section set
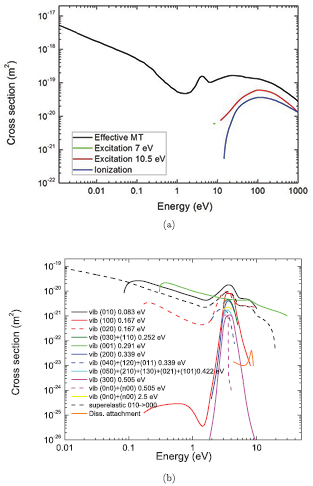
This section proposes a swarm-derived complete and consistent set of electron-neutral scattering cross sections for CO2; this will be included in the IST-Lisbon database with LXCat. The set was compiled mostly from Phelps [11] and includes 17 cross sections defined up to 1000 eV; this set describes dissociative attachment, effective momentum-transfer, eleven vibrational excitations energy losses (corresponding either to the excitation of individual levels or groups of vibrational levels), superelastic collisions with the CO2(0 1 0) vibrational state, excitation of two groups of electronic states and ionization. These cross sections are outlined in table 1 and summarized in figure 1. They assure a valid prediction of the swarm parameters when used in a two-term Boltzmann solver and for reduced electric fields below 1000 Td, as shown and discussed in section 3. Note that other research may contain interesting data for some individual processes, but not forming a complete and consistent set of cross sections. This is the case of Itikawa's compilation [12], that was not adopted as starting point for defining our dataset because it yields calculated swarm parameters strongly deviated from the experiments. Nevertheless, it provides an independent measurement for some of the processes considered over important parts of their energy range. A comparison of these cross sections with the ones proposed here is presented at the end of this section.
Figure 1. Summary of the IST-Lisbon set of electron-impact cross sections for CO2, as a function of the electron kinetic energy: effective momentum-transfer, electronic excitations and ionization (a) and vibrational excitations and dissociative attachment (b).
Download figure:
Standard image High-resolution imageTable 1. Summary of the processes considered in the cross section set proposed.
| No. | Heavy-species products | Configuration of final CO2 state | Threshold (eV) | |
|---|---|---|---|---|
| (1) | Effective momentum-transfer | CO2 | ||
| (2) | Dissociative attachment | CO + O− | ||
| (3a) | Vibrational excitation | CO2(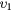 ) ) |
(0 1 0) | 0.083 |
| (3b) | Superelastic deexcitation | CO2(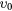 ) ) |
(0 0 0) | |
| (4a) | Vibrational excitation | CO2(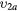 ) ) |
(0 2 0) | 0.167 |
| (4b) | Vibrational excitation | CO2(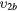 ) ) |
(1 0 0) | 0.167 |
| (5) | Vibrational excitation | CO2(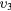 ) ) |
(0 3 0) + (1 1 0) | 0.252 |
| (6) | Vibrational excitation | CO2(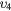 ) ) |
(0 0 1) | 0.291 |
| (7a) | Vibrational excitation | CO2(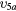 ) ) |
(2 0 0) | 0.339 |
| (7b) | Vibrational excitation | CO2(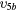 ) ) |
(0 4 0) + (1 2 0) + (0 1 1) | 0.339 |
| (8) | Vibrational excitation | CO2(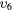 ) ) |
(0 5 0) + (2 1 0) + (1 3 0) + (0 2 1) + (1 0 1) | 0.422 |
| (9a) | Vibrational excitation | CO2(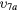 ) ) |
(3 0 0) | 0.505 |
| (9b) | Vibrational excitation | CO2(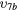 ) ) |
(0 6 0) + (2 2 0) + (1 4 0) | 0.505 |
| (10) | Vibrational excitation | CO2(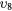 ) ) |
(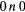 ) + ( ) + (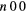 ) ) |
2.500 |
| (11) | Electronic excitation | CO2(e1) | 7.0 | |
| (12) | Electronic excitation | CO2(e1) | 10.5 | |
| (13) | Total ionization | CO |
13.3 |
A small number of modifications was made in regard to the original set by Phelps [11]. First, the cross section for superelastic collisions with the first level of the bending mode was included and should be considered as a part of the set. As a matter of fact, due to its low energy threshold (∼0.08 eV), this level presents a non-negligible population even in thermal conditions (see below), hence influencing the electron kinetics, especially at low values of the reduced electric field. Second, the cross section for the electronic excitation at 10.5 eV and the ionization initially limited to 100 eV, were extended up to 1000 eV and replaced by the total ionization cross section from Itikawa [12], respectively. Then, as the effective momentum-transfer cross section corresponds to the sum of the elastic momentum-transfer, the total excitation and the ionization cross sections, the extension of the inelastic cross sections for electron energies above 100 eV required specific modification of the effective momentum-transfer in the same energy region. Finally, the effective momentum-transfer cross section was slightly increased for electron energies below 0.1 eV, in order to compensate for the additional gain of energy associated with the superelastic collisions.
The cross sections include the eight excitations to the groups of vibrational levels schematically denoted in table 1. Electron impact vibrational excitation and de-excitation between the ground level  and level
and level  are given by reactions (3a)–(3b). This dominant de-excitation is the only superelastic process taken into account in the swarm analysis, although a second superelastic process can be taken into account for a more accurate calculation of the transport parameters at low values of the reduced electric field, typically below ∼1 Td. Reactions (4a)–(10) correspond to the electron impact vibrational excitation from the ground level
are given by reactions (3a)–(3b). This dominant de-excitation is the only superelastic process taken into account in the swarm analysis, although a second superelastic process can be taken into account for a more accurate calculation of the transport parameters at low values of the reduced electric field, typically below ∼1 Td. Reactions (4a)–(10) correspond to the electron impact vibrational excitation from the ground level  to the remaining groups of vibrational levels
to the remaining groups of vibrational levels  . In principle, processes (3a)–(9b) describe the excitation of the CO2 vibrational levels with the lowest energy thresholds. The vibrational levels associated with the energy losses
. In principle, processes (3a)–(9b) describe the excitation of the CO2 vibrational levels with the lowest energy thresholds. The vibrational levels associated with the energy losses  are clearly identified. Notably, the original cross section for
are clearly identified. Notably, the original cross section for  from [11] corresponds to the sum
from [11] corresponds to the sum  . In a recent work, Celiberto et al [23, 24] calculated new cross sections for the electron-impact resonant vibrational excitation of several transitions of the symmetric mode,
. In a recent work, Celiberto et al [23, 24] calculated new cross sections for the electron-impact resonant vibrational excitation of several transitions of the symmetric mode,  , with
, with  and
and  in the interval
in the interval  . Therefore, we have deconvoluted the cross section for
. Therefore, we have deconvoluted the cross section for  in two separate channels, as indicated in table 1, by subtracting the excitation cross section of the symmetric stretching mode (1 0 0) given in [23, 24] from the total excitation cross section by Phelps [11]. These two individual cross sections associated with
in two separate channels, as indicated in table 1, by subtracting the excitation cross section of the symmetric stretching mode (1 0 0) given in [23, 24] from the total excitation cross section by Phelps [11]. These two individual cross sections associated with  are also considered separately in [13], based on data from Biagi [25]. Furthermore, by using the spectroscopic constants reported in [4] and the energy thresholds from Phelps' set, it is possible to further suggest that the identification of the vibrational excitation generically indicated in [11] as is
are also considered separately in [13], based on data from Biagi [25]. Furthermore, by using the spectroscopic constants reported in [4] and the energy thresholds from Phelps' set, it is possible to further suggest that the identification of the vibrational excitation generically indicated in [11] as is  as follows:
as follows:  with the excitation of levels (2 0 0), (0 4 0), (1 2 0) and (0 1 1);
with the excitation of levels (2 0 0), (0 4 0), (1 2 0) and (0 1 1);  with (0 5 0), (2 1 0), (1 3 0), (0 2 1) and (1 0 1), having energy thresholds between 0.42 and 0.46 eV;
with (0 5 0), (2 1 0), (1 3 0), (0 2 1) and (1 0 1), having energy thresholds between 0.42 and 0.46 eV;  with (3 0 0), (0 6 0), (2 2 0) and (1 4 0), having energy thresholds between 0.50 and 0.51 eV, as well as contributions from other vibrational states with thresholds between 0.54 and 2.5 eV. The cross sections for the excitation of the symmetric stretching mode with
with (3 0 0), (0 6 0), (2 2 0) and (1 4 0), having energy thresholds between 0.50 and 0.51 eV, as well as contributions from other vibrational states with thresholds between 0.54 and 2.5 eV. The cross sections for the excitation of the symmetric stretching mode with  and
and  were separated from the cross sections for the other excitation modes, using the same procedure as for
were separated from the cross sections for the other excitation modes, using the same procedure as for  and the data from [23, 24], as indicated in table 1. An additional deconvolution of processes with
and the data from [23, 24], as indicated in table 1. An additional deconvolution of processes with  and the addition of cross sections for other vibrational excitations may lead to an improvement of the swarm analysis and would certainly be significant in gaining a more complete insight into CO2 plasmas. On-going work by Celiberto, Laporta and co-workers may provide invaluable data to pursue this task [23, 24].
and the addition of cross sections for other vibrational excitations may lead to an improvement of the swarm analysis and would certainly be significant in gaining a more complete insight into CO2 plasmas. On-going work by Celiberto, Laporta and co-workers may provide invaluable data to pursue this task [23, 24].
Reactions (11)–(12) generically represent the excitation of electronic states. We believe that the corresponding cross sections describe various processes, including CO2 dissociation. Polak and Slovetsky [17] refer a group of states with energy thresholds ≈8 eV, the triplets  ,
,  ,
,  and
and  and the singlets
and the singlets  ,
,  and
and  . The same states are tabulated by Itikawa [12], who indicates energy thresholds in the range 8.15–9.32 eV as given by [27], although an energy threshold as low as 4.9 eV is given by Rabalais et al [29] for the lowest laying state
. The same states are tabulated by Itikawa [12], who indicates energy thresholds in the range 8.15–9.32 eV as given by [27], although an energy threshold as low as 4.9 eV is given by Rabalais et al [29] for the lowest laying state  [12]. The same states are also identified in [26, 28], where an energy threshold of 7.35 eV is given for the
[12]. The same states are also identified in [26, 28], where an energy threshold of 7.35 eV is given for the  state. A second group of states, with energy thresholds of about 11 eV is identified in [12, 27, 28],
state. A second group of states, with energy thresholds of about 11 eV is identified in [12, 27, 28],  ,
,  and
and  . The two groups of electronically excited states described are very likely included in reactions (11) and (12), respectively, but the separation of the lumped cross sections into individual processes seems to presently be beyond reach. Moreover, these cross sections may contain other mechanisms and the possible roles of these states in CO2 dissociation is far from clear. An assessment of dissociation is carried out in section 4, the reader should refer to this section for further details. Notably, in [13] the two electronic excitation cross sections are assigned in a simplified way to the excitation of the lowest state in each group,
. The two groups of electronically excited states described are very likely included in reactions (11) and (12), respectively, but the separation of the lumped cross sections into individual processes seems to presently be beyond reach. Moreover, these cross sections may contain other mechanisms and the possible roles of these states in CO2 dissociation is far from clear. An assessment of dissociation is carried out in section 4, the reader should refer to this section for further details. Notably, in [13] the two electronic excitation cross sections are assigned in a simplified way to the excitation of the lowest state in each group,  and
and  .
.
The non-conservative processes considered are ionization, described by reaction (13), and dissociative attachment, represented by reaction (2). The maximum value of the dissociative attachment cross section is not very high (∼10−22 m2), so in general it does not contribute significantly to CO2 decomposition [13]. However, it may be important for discharge maintenance by affecting the charged particle balance.
It is worth noting that the superelastic process in (3b) is considered using the Klein–Rosseland relation and the vibrational excitation cross section at 0.083 eV. When the population of the vibrationally excited states is high enough, the excited molecules can effectively transfer energy back to the electrons. The low energy threshold of the (0 1 0) state makes it important to account for superelastic collisions even for gas temperatures around room temperature,  K, since the relative population of this state is already about 8%. The energy gained in these processes is especially noticeable at low reduced electric fields, while for
K, since the relative population of this state is already about 8%. The energy gained in these processes is especially noticeable at low reduced electric fields, while for  Td (and
Td (and  K) the EEDF is barely affected by superelastic mechanisms (see section 3).
K) the EEDF is barely affected by superelastic mechanisms (see section 3).
It is instructive to compare the current cross sections with the data presented in Itikawa's compilation [12] for some individual processes over specific energy regions. Figure 2 shows this comparison for the elastic momentum-transfer and the excitation of the (0 1 0), (0 0 1) and (1 0 0) vibrational levels. As noted above, the IST-Lisbon elastic momentum-transfer can be obtained by subtracting from the effective momentum-transfer (shown in figure 1(a)) the contributions due to vibrational and electronic excitation and ionization. The cross sections differ for energies above ∼3 eV (figure 2(a)). This difference produces relatively small but visible deviations in the calculated reduced mobility, of about 5% and 20%, respectively for E/N < 10 Td and E/N > 50 Td, with a better agreement between the Boltzmann calculations and the measured swarm data obtained when the cross section proposed in this work is used. The vibrational excitation cross sections presented in [12] were obtained by extrapolating the beam-data experimental differential cross sections of Kitajima et al [30] to obtain integral cross sections, with a reported uncertainty of 30%. For the excitations of the (0 1 0) and (0 0 1) levels, the cross sections exhibit some evident differences to the ones proposed here, but remain relatively similar in magnitude and shape with regard to the available energy ranges, and are nearly always within the experimental uncertainty (figure 2(b)). On the contrary, Itikawa's recommended cross section corresponding to the (1 0 0) mode differs significantly from the calculated cross section proposed in this work (figure 2(c)). Notice, however, that the (1 0 0) cross section is surrounded by significant uncertainty and the beam data cross section should be used with caution and is difficult to compare with swarm derived cross sections, as pointed out in [12], due to its smaller magnitude and to the Fermi resonance between the (1 0 0) and (0 2 0) levels (so that the dyad cannot be separated in swarm experiments). Regarding the other mechanisms, ionization is taken from [12], attachment from Phelps [11] is the same as in [12], whereas dissociation is discussed separately in section 4.
Figure 2. Comparison between the IST-Lisbon and Itikawa's cross sections corresponding to: elastic momentum-transfer (a); vibrational excitations (0 1 0) and (0 0 1) (b) and (1 0 0) (c).
Download figure:
Standard image High-resolution imageThe cross section set presented in this section will be soon available in the IST-Lisbon database with LXCat [31]. At present, the IST-Lisbon database includes complete and consistent sets of electron scattering cross sections for argon, helium, nitrogen, oxygen, hydrogen and methane, issued by the Group of Gas Discharges and Gaseous Electronics with Instituto de Plasmas e Fusão Nuclear, Instituto Superior Técnico, Lisboa, Portugal.
3. Swarm calculations
In this section the set of electron-impact cross sections for CO2 proposed here is validated from the comparison between calculated and measured values of electron transport parameters, namely the reduced mobility,  , the characteristic energy,
, the characteristic energy,  (DT is the transverse diffusion coefficient), the reduced longitudinal diffusion coefficient,
(DT is the transverse diffusion coefficient), the reduced longitudinal diffusion coefficient,  , and the reduced Townsend effective ionization coefficient,
, and the reduced Townsend effective ionization coefficient,  . The calculations are performed using the freeware package BOLSIG+, a numerical solver based on the two-term approximation of the electron Boltzmann equation [22], and are compared to the measurements available in several databases of the LXCat open-access website [32–35]. All calculations are made using three sets of cross sections: the IST-Lisbon set proposed in this work; the Phelps set [11, 36]; and a set obtained by excluding the superelastic cross section from IST-Lisbon.
. The calculations are performed using the freeware package BOLSIG+, a numerical solver based on the two-term approximation of the electron Boltzmann equation [22], and are compared to the measurements available in several databases of the LXCat open-access website [32–35]. All calculations are made using three sets of cross sections: the IST-Lisbon set proposed in this work; the Phelps set [11, 36]; and a set obtained by excluding the superelastic cross section from IST-Lisbon.
Since the current set was developed to be used in a two-term Boltzmann solver, it should not be used in conditions where the anisotropic corrections become too significant. In practice, taking into account the precision in the power balance, the magnitude of the anisotropic correction and the agreement with the experimental swarm data, the current set can be used safely in any Boltzmann solver for reduced electric fields lower than 1000 Td and using an energy grid extending up to 1000 eV. In other cases multi-term expansions or Monte Carlo methods accounting for the anisotropy of the cross sections should be used. An example of a freely available software accounting for this anisotropy is the MagBoltz code by Stephen Biagi [37–39]. Work is in progress to evaluate and compare different techniques to solve the electron Boltzmann equation using the proposed cross section set [40].
In typical conditions of swarm experiments, the electron transport parameters are functions essentially of the local reduced electric field strength, E/N. However, dependences on the gas temperature, Tg, may be evidenced at low values of E/N, usually for ∼1–10 Td and below [41]. As anticipated in the previous section, this is also the case of CO2. The dependence is mainly an outcome of the superelastic collisions with vibrationally excited molecules in the (0 1 0) bending mode, whose population depends on Tg. Notice that swarm experiments correspond typically to conditions of negligible vibrational excitation by electron impact, so that the populations of the vibrationally excited states can be assumed to follow a Boltzmann distribution at the gas temperature. In any case, at low E/N the plasma tends to a thermal equilibrium situation, with the electron temperature similar to the gas temperature. The previous considerations imply that the population of the (0 1 0) vibrationally excited state (with 0.083 eV excitation energy and statistical weight of 2) must be given as input parameter to the code. For guidance, the Boltzmann relative population of this level at  K is 0.076.
K is 0.076.
In principle, the electron swarm parameters may depend on the way the electron density is changing along the swarm. Since the measurements can adopt different conditions and configurations, the choice of the growth model included in the calculations may be relevant for the purpose of comparing the calculations with the experimental data. The calculations presented in this work were performed assuming one of the following situations: 'steady-state Townsend' (SST), which considers an exponential growth of the electron current between the electrodes; or 'pulsed Townsend' (PT), in which the spatially averaged electron number density increases exponentially in time. The SST formulation was used for the calculations of  , uk and
, uk and  . Conversely, the results for
. Conversely, the results for  were obtained using the PT model, because the longitudinal diffusion cannot be measured in SST experiments [42]. At low E/N, the results are unaffected by the choice of the growth model, since the rate of production of new electrons is sufficiently low and therefore, the electron number density is approximately constant. The values of
were obtained using the PT model, because the longitudinal diffusion cannot be measured in SST experiments [42]. At low E/N, the results are unaffected by the choice of the growth model, since the rate of production of new electrons is sufficiently low and therefore, the electron number density is approximately constant. The values of  , uk and of
, uk and of  , calculated using the SST and PT models, differ by 1% and 5% at 200 Td, respectively, the differences increasing to 10% and 15% for higher E/N.
, calculated using the SST and PT models, differ by 1% and 5% at 200 Td, respectively, the differences increasing to 10% and 15% for higher E/N.
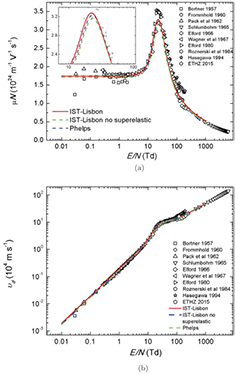
Figure 3 presents the calculated and measured values of the reduced mobility and drift velocity  , the latter being the parameter measured in swarm experiments. The measurements are taken from the Dutton, LAPLACE and ETHZ databases [43–61]. All the calculations are in reasonable agreement with the measurements over the full range of values of E/N. As demonstrated, both IST-Lisbon and Phelps cross section sets consistently reproduce the reduced mobility for the lower values of E/N, within 1% of the measurements. This is mainly due to the adequate choice of the effective momentum-transfer cross sections in these sets. The influence of superelastic collisions is also clearly visible, revealing that Phelps' set would give worse results if superelastic collisions were added, thus suggesting that this complete set was deduced to fit measurements at
, the latter being the parameter measured in swarm experiments. The measurements are taken from the Dutton, LAPLACE and ETHZ databases [43–61]. All the calculations are in reasonable agreement with the measurements over the full range of values of E/N. As demonstrated, both IST-Lisbon and Phelps cross section sets consistently reproduce the reduced mobility for the lower values of E/N, within 1% of the measurements. This is mainly due to the adequate choice of the effective momentum-transfer cross sections in these sets. The influence of superelastic collisions is also clearly visible, revealing that Phelps' set would give worse results if superelastic collisions were added, thus suggesting that this complete set was deduced to fit measurements at  K only. It is also worth noting that the IST-Lisbon calculated peak of the reduced mobility, at
K only. It is also worth noting that the IST-Lisbon calculated peak of the reduced mobility, at  20 Td, fits very well the recent measurements provided by ETHZ [33].
20 Td, fits very well the recent measurements provided by ETHZ [33].
Figure 3. Measured and calculated reduced electron mobility (a) and drift velocity (b) in CO2 at  K, as a function of E/N. The symbols are experimental data (see text) and the lines are calculation results obtained using a two-term Boltzmann solver with the following cross section sets: IST-Lisbon with (
K, as a function of E/N. The symbols are experimental data (see text) and the lines are calculation results obtained using a two-term Boltzmann solver with the following cross section sets: IST-Lisbon with ( ) and without (
) and without ( ) the superelastic process included; and Phelps (
) the superelastic process included; and Phelps ( ). The insert in (a) is a zoom over the peak region of the reduced electron mobility.
). The insert in (a) is a zoom over the peak region of the reduced electron mobility.
Download figure:
Standard image High-resolution imageThe influence of superelastic collisions with CO2 (010) is further illustrated in figures 4(a) and (b), which show the EEDFs calculated at 1 Td and 50 Td, respectively, with and without the inclusion of the superelastic process. The effect can be seen in both cases, even if superelastic collisions are not very important at higher reduced electric fields. This is well-known from studies in other gases, noticeably nitrogen [62], since for high values of E/N the electrons gain most of their energy from the electric field. A detailed study of the influence of superelastic collisions in CO2 was recently published in [14, 63].
Figure 4. Electron energy distribution function in CO2 obtained with the IST-Lisbon cross section set for 1 Td (a) and 50 Td (b), with ( ) and without (- - - -) the superelastic process included.
) and without (- - - -) the superelastic process included.
Download figure:
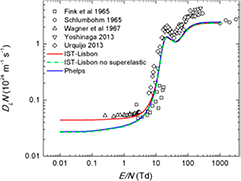
Standard image High-resolution imageFigure 5 shows the calculated and measured reduced longitudinal diffusion coefficient,  . The IST-Lisbon cross section set yields good agreement (within 7%) between the two-term Boltzmann calculations and the measurements by Wagner et al [57], at low E/N. The inclusion of the superelastic mechanism (3b) is mainly responsible for the differences of ∼40% observed between the results of the various datasets, for E/N < 10 Td.
. The IST-Lisbon cross section set yields good agreement (within 7%) between the two-term Boltzmann calculations and the measurements by Wagner et al [57], at low E/N. The inclusion of the superelastic mechanism (3b) is mainly responsible for the differences of ∼40% observed between the results of the various datasets, for E/N < 10 Td.
Figure 5. Measured and calculated reduced longitudinal diffusion coefficient in CO2 at  K, as a function of E/N. The symbols are experimental data (see text) and the lines are calculations using a two-term Boltzmann solver. The cross section sets used in the calculations are IST-Lisbon with (
K, as a function of E/N. The symbols are experimental data (see text) and the lines are calculations using a two-term Boltzmann solver. The cross section sets used in the calculations are IST-Lisbon with ( ) and without (
) and without ( ) the superelastic process included; and Phelps (
) the superelastic process included; and Phelps ( ).
).
Download figure:
Standard image High-resolution imageThe ratio of the transversal electron diffusion coefficient to the electron mobility is a measurable quantity with dimensions of energy expressed in eV, known as the characteristic energy, uk. As it is well-known, when the EEDF is Maxwellian uk = kT/e, where T is the electron temperature, k the Boltzmann constant and e the electron charge. In other cases there is no simple relationship between the average and the characteristic energies. Nevertheless,  provides a very useful energy scaling, since the average electron energy is not easily accessible from experiments. The comparison between the calculated and measured values of
provides a very useful energy scaling, since the average electron energy is not easily accessible from experiments. The comparison between the calculated and measured values of  as a function of E/N is shown in figure 6, for two values of the gas temperature, 195 and 300 K. The results strikingly expose the importance of superelastic collisions. In fact, while the IST-Lisbon cross section set gives uk in good agreement with the measurements for both values of the gas temperature, Phelps' set yields predictions at 300 K that incorrectly match the experimental data measured at 195 K by Warren et al [58]. The same occurs when superelastic collisions are excluded from the IST-Lisbon set. The gas temperature affects uk at very low E/N, in a situation where the electrons gain a considerable amount of energy in elastic and superelastic collisions with the target molecules. Accordingly, the inclusion of superelastic collisions yields characteristic energies in significantly better agreement with the measurements. In the region E/N < 10 Td, the predictions differ from the measurements by 8% at 195 K and by 5% at 300 K. The only available data for E/N > 100 Td, measured by Schlumbohm [52, 53], deviates significantly (by a factor of 2) from the calculations made with either sets.
as a function of E/N is shown in figure 6, for two values of the gas temperature, 195 and 300 K. The results strikingly expose the importance of superelastic collisions. In fact, while the IST-Lisbon cross section set gives uk in good agreement with the measurements for both values of the gas temperature, Phelps' set yields predictions at 300 K that incorrectly match the experimental data measured at 195 K by Warren et al [58]. The same occurs when superelastic collisions are excluded from the IST-Lisbon set. The gas temperature affects uk at very low E/N, in a situation where the electrons gain a considerable amount of energy in elastic and superelastic collisions with the target molecules. Accordingly, the inclusion of superelastic collisions yields characteristic energies in significantly better agreement with the measurements. In the region E/N < 10 Td, the predictions differ from the measurements by 8% at 195 K and by 5% at 300 K. The only available data for E/N > 100 Td, measured by Schlumbohm [52, 53], deviates significantly (by a factor of 2) from the calculations made with either sets.
Figure 6. Measured and calculated characteristic energy in CO2 as a function of E/N. The symbols are experimental data (see text). The lines are calculation results obtained using a two-term Boltzmann solver at different temperatures, with the following cross section sets: IST-Lisbon 195 K ( ) and 300 K with (
) and 300 K with ( ) and without (
) and without ( ) the superelastic process included; and Phelps 300 K (
) the superelastic process included; and Phelps 300 K ( ).
).
Download figure:
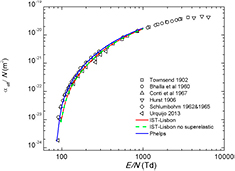
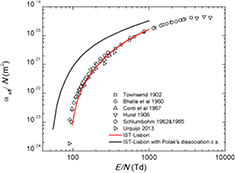
Standard image High-resolution imageThe reduced Townsend ionization coefficient,  , is defined as the number of ionization events per unit distance in the drift direction, normalized to the neutral number density. Figure 7 compares calculations and measurements of the reduced effective ionization coefficient, defined as
, is defined as the number of ionization events per unit distance in the drift direction, normalized to the neutral number density. Figure 7 compares calculations and measurements of the reduced effective ionization coefficient, defined as  , where η is the corresponding attachment coefficient. The contribution of electron attachment is noticeable only at the lower values of electric field, around 100 Td. All sets tested here are able to reproduce fairly well the measured
, where η is the corresponding attachment coefficient. The contribution of electron attachment is noticeable only at the lower values of electric field, around 100 Td. All sets tested here are able to reproduce fairly well the measured  (within 2%) for E/N < 200 Td, yielding results that deviate up to 40%, for higher E/N.
(within 2%) for E/N < 200 Td, yielding results that deviate up to 40%, for higher E/N.
Figure 7. Calculated and measured reduced effective ionization coefficient versus the reduced electric field (E/N) in CO2. The lines are results from two-term Boltzmann calculations corresponding to the calculations made with the cross sections from IST-Lisbon with ( ) and without (
) and without ( ) the superelastic process included; and Phelps (
) the superelastic process included; and Phelps ( ). The symbols are experimental data (see text).
). The symbols are experimental data (see text).
Download figure:
Standard image High-resolution image4. Dissociation of CO2
The growing interest in the plasma assisted conversion of greenhouse gases (mainly CO2 and CH4) into chemical compounds and liquid fuels has brought attention to the complexity of CO2 dissociation, since it corresponds to the limiting step for achieving an efficient energy storage. The dissociation of CO2 is a strongly endothermic process, which makes non-equilibrium plasma technologies promising candidates for defining an energy-efficient reaction path [6, 7]. The great potential of non-equilibrium plasmas is due to the presence of energetic electrons that can initiate chemical reactions unattainable by ordinary thermal mechanisms, e.g. through vibrational ladder-climbing processes. Indeed, an efficient dissociation path can be induced by electrons with energies of only ∼1 eV, by transferring energy to the asymmetric stretching mode of the CO2 molecule [4]. Under favourable conditions the vibrational quanta can be pumped-up to the dissociation limit during the relaxation process, due to non-resonant vibration-vibration energy exchanges.
These considerations are at the basis of the considerable work conducted in the 1970s and 1980s [4, 64–67], aiming to use vibrational energy to achieve the dissociation process rather than promoting the direct electron impact process, with the belief that the activation energy of the former is much smaller than that of the latter. Nevertheless, dissociation through electronic excitation can be a dominant mechanism in some plasmas, for example in non-equilibrium plasmas with high values of reduced electric fields, such as dielectric barrier discharges or when plasma is generated by degradation of very energetic particles. Either way, in order to optimize plasma-assisted conversion of CO2, a profound knowledge of all dissociative channels is needed, including the direct route by electron impact. Surprisingly, the CO2 dissociation cross section by electron impact is poorly understood. As a matter of fact, the cross sections for the dissociation of CO2 through electronic excitation are reported by several authors, but they vary significantly both in magnitude and shape. Notably, there are many unknowns regarding this issue.
The cross section set proposed in this work contains two electronic excitation cross sections, with thresholds at 7 eV and 10.5 eV, both originating from the Phelps database. Hake and Phelps derived the original cross sections from a swarm analysis [20], and were later modified by Lowke and Phelps [11] in accordance with newer measurements. Since the 10.5 eV cross section in [11] is limited to 100 eV, we included an extension up to 1 keV, as stated in section 2. To the best of our knowledge, the two electronic excitation cross sections do not correspond to any specific process, but rather to a combination of mechanisms lumped as a global energy loss, including the excitation of levels  ,
,  ,
,  ,
,  ,
,  ,
,  and
and  around 8–9 eV,
around 8–9 eV,  ,
,  and
and  around 11 eV, as described in section 2. These excitation levels most likely represent dissociative channels, but they may contain more than just dissociation.
around 11 eV, as described in section 2. These excitation levels most likely represent dissociative channels, but they may contain more than just dissociation.
Several authors have identified the process with the threshold at 7 eV as the channel of CO2 dissociation. One recent example is the work of Pietanza et al [14], which considers the 7 eV cross section from the Phelps database as the dissociative channel, while the 10.5 eV cross section is assumed to correspond to electronic excitation without dissociation. This standpoint is also adopted in the work of Wiegand et al [19], where the measured dissociation rate in the CO2 laser gas mixture is reported to be in agreement with the measurements of Corvin and Corrigan [15] and the calculations of Nighan [68] performed with the 7 eV Phelps cross section. Capezzuto et al [18] acknowledges the research efforts by Wiegand and Nighan, considering that the same cross section corresponds to the electron-impact dissociative excitation
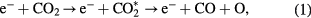
and used it to calculate the rate coefficient of CO2 dissociation. Comparing the calculations of Wiegand and Nighan to their measurements, Capezzuto and co-workers conclude that equation (1) and the 7 eV cross section cannot represent the sole mechanism of CO2 dissociation.
Cross sections of CO2 dissociation are also offered by Corvin and Corrigan [15]. However, what was measured was not the cross section, but the dissociation rate coefficient. Then, assuming a Maxwellian EEDF, they estimated a cross section with a threshold at 6.1 eV, constructed in order to give a rate coefficient in agreement with their own measurements.
Cosby and Helm [21] measured the absolute cross section for the dissociation of CO2, using electron beam techniques. They found a higher threshold, near  eV, corresponding to two dissociation channels:
eV, corresponding to two dissociation channels:
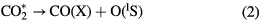
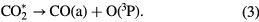
According to their predictions, O(1S) and CO(a) represent 73 and 27
and 27 , respectively, of the dissociation products. In his compilation of CO2 cross sections [12], Itikawa includes the dissociation cross section leading to the production of O(1S) measured by LeClair and McConkey [16]. The cross section is obtained by measuring the radiation of the neutral fragment using a solid Xe detector. They conclude that besides (2) many other channels contribute to the production of O(1S). The cross sections compiled by Itikawa are taken as representative of the total dissociation in [5, 13, 69–73].
, respectively, of the dissociation products. In his compilation of CO2 cross sections [12], Itikawa includes the dissociation cross section leading to the production of O(1S) measured by LeClair and McConkey [16]. The cross section is obtained by measuring the radiation of the neutral fragment using a solid Xe detector. They conclude that besides (2) many other channels contribute to the production of O(1S). The cross sections compiled by Itikawa are taken as representative of the total dissociation in [5, 13, 69–73].
In a very thorough work published in 1976, Polak and Slovetsky developed a method of computing the dissociation cross sections giving rise to the production of neutral species [17]. Using this method, they calculated the electron-impact dissociation cross sections for hydrogen, nitrogen, oxygen, nitric oxide, carbon monoxide, CO2, methane and other saturated hydrocarbons, stressing the importance of a complex mechanism involving a multiplicity of channels leading to dissociation. Regarding CO2, they address three channels represented by the following cross sections:
- (i)cross section of dissociation with formation of the CO(a) molecule;
- (ii)cross section of dissociation by excitation of allowed transitions from
 –9 eV states;
–9 eV states; - (iii)cross section of dissociation by excitation of forbidden transitions from
 –9 eV states.
–9 eV states.
In their work it is stated that about 40% of the cross section (i) corresponds to the direct formation of CO(a) molecules, the remaining representing cascade transitions from higher triplet states of the CO molecule, resulting also from CO2 dissociation. The excitation cross section (ii) of levels to which optical transitions are allowed, observed at  7–9 eV during light absorption, are computed from the absorption spectra. In what follows, the cross section (iii) is ignored, as it is much smaller than (i) and (ii). The total dissociation cross section from [17] (i.e. the sum of (i), (ii) and (iii)) differs significantly from Corvin's cross section, both in shape and magnitude (see figure 8).
7–9 eV during light absorption, are computed from the absorption spectra. In what follows, the cross section (iii) is ignored, as it is much smaller than (i) and (ii). The total dissociation cross section from [17] (i.e. the sum of (i), (ii) and (iii)) differs significantly from Corvin's cross section, both in shape and magnitude (see figure 8).
Figure 8. Cross sections for the electron-impact dissociation of CO2, proposed by different authors.
Download figure:
Standard image High-resolution imageThe work by Polak and Slovetsky gives a very interesting framework to analyse the problem. On the one hand, dissociation cross sections are calculated as the result of individual excitations of different electronically excited states, not through the measurement of excited products (which accounts for only a fraction of dissociation) or lumped energy losses. On the other hand, their method was applied to several other molecules with success, which gives some confidence to use these cross sections as starting point for CO2. The identification in [17] of two groups of states, one with energy thresholds around 7–9 eV and the other around 11 eV, may suggest an association of these excitations with the cross sections at 7 and 10.5 eV from the Phelps and the IST-Lisbon datasets. The comparison of the cross sections (i) and (ii) with those proposed here for the electronic excitations is given in figure 8, revealing some similarity in shape, but a much smaller magnitude for the data in [17]. These results give a first suggestion that the 7 eV and 10.5 eV cross sections probably include more than just dissociation. For completeness, figure 8 depicts as well the cross sections proposed in [12, 15, 21].
The rate coefficient of CO2 dissociation can be calculated as a function of the reduced electric field according to
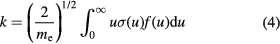
where me is the electron mass, f(u) is the EEDF defined so that  and
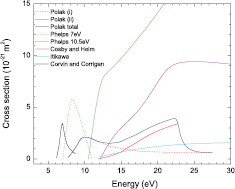
and  is the cross section for dissociation by electron impact. Herein, the dissociation rate coefficient is calculated from (4) using the cross sections reported by Corvin and Corrigan [15], Polak and Slovetsky [17], Cosby and Helm [21], Itikawa [12] and Phelps electronic excitation [11] (very similar to ours), with an EEDF obtained using the IST-Lisbon cross sections proposed in this work. The results are shown in figure 9. It can be seen that the 7 eV Phelps cross section and the Polak and Slovetsky total cross section leads to rate coefficients with comparable values, especially for
is the cross section for dissociation by electron impact. Herein, the dissociation rate coefficient is calculated from (4) using the cross sections reported by Corvin and Corrigan [15], Polak and Slovetsky [17], Cosby and Helm [21], Itikawa [12] and Phelps electronic excitation [11] (very similar to ours), with an EEDF obtained using the IST-Lisbon cross sections proposed in this work. The results are shown in figure 9. It can be seen that the 7 eV Phelps cross section and the Polak and Slovetsky total cross section leads to rate coefficients with comparable values, especially for  Td. Corvin's cross section yields a rate coefficient in reasonably good agreement with their experimental measurements, but this is of little significance since it just reflects the way the cross section was derived. It is also interesting to note that the three cross sections assigned to dissociation with the formation of excited products, the calculations by Polak and Slovetskii (i) and the later measurements by Itikawa and by Cosby and Helm, all give results within the same order of magnitude for
Td. Corvin's cross section yields a rate coefficient in reasonably good agreement with their experimental measurements, but this is of little significance since it just reflects the way the cross section was derived. It is also interesting to note that the three cross sections assigned to dissociation with the formation of excited products, the calculations by Polak and Slovetskii (i) and the later measurements by Itikawa and by Cosby and Helm, all give results within the same order of magnitude for  Td. This reinforces the idea that the cross sections from [17] establish an interesting starting point to analyse the question. For instance, Phelp's cross section at 10.5 eV clearly overestimates dissociation with the formation of excited products. Moreover, the 7 eV cross section seems also to overestimate dissociation at higher values of E/N.
Td. This reinforces the idea that the cross sections from [17] establish an interesting starting point to analyse the question. For instance, Phelp's cross section at 10.5 eV clearly overestimates dissociation with the formation of excited products. Moreover, the 7 eV cross section seems also to overestimate dissociation at higher values of E/N.
Figure 9. Dissociation rate coefficient of CO2 as a function of E/N, calculated using different dissociation cross sections. The symbols represent the measurements by Corvin and Corrigan.
Download figure:
Standard image High-resolution imageAlthough the calculations are compared with a single set of experimental measurements, the results suggest that the 7 eV and 10.5 eV cross sections are likely to include more than dissociation. To test this hypothesis, the swarm analysis was repeated for the IST-Lisbon set, but this time replacing the electronic excitation cross sections at 7 eV and 10.5 eV with those of Polak and Slovetsky (ii) and (i). The resulting cross section set fails to predict the expected behaviour of the reduced Townsend effective ionization coefficient, as shown in figure 10. This constitutes an additional confirmation that some electron energy losses are missing in the cross sections from [17], probably not ascribable to dissociation, making it delicate to directly assign any of the electronic excitation cross sections from Phelps or IST-Lisbon datasets to dissociation.
Figure 10. Calculated and measured reduced effective ionization coefficient in CO2, as a function of E/N. The symbols represent experimental data (see text). The lines are calculation results obtained using a two-term Boltzmann solver with the following cross section sets: IST-Lisbon ( ); IST-Lisbon, replacing the electronic excitation cross sections at 7 eV and 10.5 eV with Polak's (i) and (ii) (
); IST-Lisbon, replacing the electronic excitation cross sections at 7 eV and 10.5 eV with Polak's (i) and (ii) ( ).
).
Download figure:
Standard image High-resolution imageTaking into account the information available to date, namely: (a) the acceptable agreement between the dissociation rates calculated using Polak and Slovetsky's cross sections (i) and the later measurements adopted in Itikawa for the same processes; (b) that the cross sections in [17] are based on a theory that predicts an additional channel which currently remains difficult to assess experimentally (as the products are not excited); and (c) that the theory in [17] was also applied with success to other molecules; we currently suggest using the IST-Lisbon set to calculate the EEDF and the electron transport parameters, and later integrating Polak and Slovetsky's total cross section with the calculated EEDF in order to obtain a first estimation for the dissociation rate. Further theoretical and experimental work is desirable, investigating the influence of the different cross section sets on dissociation and the validity of this recommendation.
5. Final remarks
This paper proposes a swarm-derived complete and consistent electron-impact cross section set for CO2 to be included in the IST-Lisbon database at LXCat, deduced from an improvement to the Phelps database [11]. Superelastic collisions with CO2 (0 1 0) molecules are considered in this work, as it is clearly shown to be important with regard to energy transfers between electrons and CO2 molecules, at low reduced electric fields. Additionally, an effort was made to deconvolute the original vibrational excitation cross sections.
The new IST-Lisbon set yields accurate predictions of the characteristic energy giving the correct dependences with the gas temperature; this is very evident for E/N < 10 Td and is impossible to obtain if superelastic collisions are not taken into account. The proposed cross sections are able to produce reduced electron mobilities that are in good agreement with the measured data within a large range of E/N values, as well as good predictions of the reduced Townsend ionization coefficient.
This work addresses as well the problems associated with CO2 dissociation, reviewing the available electron-impact dissociation cross sections. In particular, the dissociation coefficient is calculated upon integration of the EEDFs obtained using the IST-Lisbon set, over the different cross sections associated with dissociation available in the literature. The results strongly suggest that our cross sections for electronic excitation, based on Phelps [11] and with energy thresholds at 7 eV and 10.5 eV, most likely include not only dissociative channels but also some additional contributions.
Despite the numerous studies on CO2, electron-impact dissociation is still not completely understood, with different authors making different assumptions and using different cross sections to calculate the dissociation rates. We consider that the cross sections calculated by Polak and Slovetsky [17] address (i) the formation of O(3P) + CO(a) and (ii) the dissociation by excitation of allowed transitions, and provide a good basis for understanding and analysing the problems of CO2 dissociation. As a matter of fact, these cross sections give dissociation rate coefficients through mechanism (i) similar to the ones obtained using the experimental cross section reported in [12] and total dissociation rates in reasonable agreement with the measurements from [15].
At present, we suggest using Polak and Slovetski's cross section [17] to calculate the dissociation rate coefficient of CO2, upon integration of a previously calculated EEDF. However, this cross section should not be used in a Boltzmann solver to calculate the EEDF nor it is part of the IST-Lisbon dataset. The use of the electronic excitation at 7 eV may provide a simpler and reasonable alternative to this procedure. Notice that the cross sections from [17] produce a dissociation rate coefficient comparable to the one calculated with the Phelps 7 eV cross section at  Td, which is the reduced field favouring the indirect dissociation route through vibrational excitation.
Td, which is the reduced field favouring the indirect dissociation route through vibrational excitation.
Research aims to verify the influence of the different cross sections in the overall kinetics of CO2 plasmas in different types of discharges, namely by testing the influence of the cross section set proposed in this work and of the cross sections for dissociation assumed by different authors in the models of the PLASMANT group in Antwerpen [74]. Preliminary results seem to indicate that Polak and Slovetski's cross section may lead to a dissociation rate that is too low and that the 7 eV excitation may provide better results. Further work is needed in order to clarify this question and to define a dissociation cross section for CO2. In addition, a deconvolution of current vibrational excitation cross sections and/or the inclusion of additional vibrational excitation channels would also contribute to improve our knowledge of the energy transfer made in CO2.
Acknowledgments
We are indebted to Roberto Celiberto and Vincenzo Laporta for providing state-to-state cross sections for vibrational excitation. We would like to thank Annemie Bogaerts for very fruitful discussions. This work was partially supported by the Portuguese FCT—Fundação para a Ciência e a Tecnologia, under Projects UID/FIS/50010/2013 and PTDC/FIS-PLA/1420/2014 (PREMiERE), and the grant PD/BD/105884/2014 (PD-F APPLAuSE).