Abstract
The suitability of atomic layer deposited (ALD) titanium oxide (TiOx) as a top gate dielectric and passivation layer for indium gallium zinc oxide (InGaZnO115) ion sensitive field effect transistors (ISFETs) is investigated. TiOx is an attractive barrier material, but reports of its use for InGaZnO thin film transistor (TFT) passivation have been conflicting thus far. In this work, it is found that the passivated TFT's behavior depends on the TiOx deposition temperature, affecting critical device characteristics such as threshold voltage, field-effect mobility and sub-threshold swing. An O2 annealing step is required to recover TFT performance post passivation. It is also observed that the positive bias stress response of the passivated TFTs improves compared the original bare device. Secondary ion mass spectroscopy excludes the effects of hydrogen doping and inter-diffusion as sources of the temperature-dependent performance change, therefore indicating that oxygen gettering induced by TiOx passivation is the likely source of oxygen vacancies and, consequently, carriers in the InGaZnO film. It is also shown that potentiometric sensing using ALD TiOx exhibits a near Nernstian response to pH change, as well as minimizes VTH drift in TiOx passivated InGaZnO TFTs immersed in an acidic liquid. These results add to the understanding of InGaZnO passivation effects and underscore the potential for low-temperature fabricated InGaZnO ISFETs to be used as high-performance mobile chemical sensors.
Export citation and abstract BibTeX RIS
1. Introduction
Indium gallium zinc oxide (InGaZnO) has been the subject of much research in recent years due to its attractive properties, such as high electron mobility when deposited at room temperature and high transparency at visible wavelengths, making it an excellent semiconductor material for thin film transistors (TFTs) in high-resolution and flexible flat panel displays [1]. These traits also make InGaZnO TFTs highly attractive candidates for flexible and/or wearable sensor applications, in particular if they can be deployed as high-performance ion sensitive field effect transistors (ISFETs). The original ISFET, first demonstrated by Bergveld using a silicon n-channel FET with a SiO2 top gate dielectric [2, 3], possesses a fundamental upper bound on its sensitivity, known as the Nernst Limit, corresponding to a maximum shift of 59 mV of the threshold voltage (VTH) per unit pH change at room temperature. The fact that these devices can be produced using CMOS-compatible processes, with some additional post-processing, has led to high levels of system integration and, in some cases, advanced biological sensing applications [4, 5].
To overcome the Nernst Limit, a novel approach to pH sensing involving double-gated (DG) TFTs has been under investigation in recent years. Organic [6], ZnO [7], and InGaZnO [8–10] DG-TFTs have all been reported. Hybrid InGaZnO DG-TFTs with an extended gate have also been shown [11, 12]. Though sensitivities as high as 2.25 V pH−1 have been measured [7], all of these investigations share a common shortcoming: they rely on the use of a thick, thermally grown SiO2 bottom gate dielectric. This means that neither transparent glass, nor flexible plastics can be used as substrates. Only recently has a fully-transparent and low-temperature InGaZnO DG-TFT on glass been reported [13]. In addition to these works, it should also be noted that a recent study claimed 'Super-Nernstian' pH sensitivity using an unpassivated InGaZnO DG-TFT [14]. Since it is well known that even highly diluted acidic solutions can be used to etch InGaZnO [15], the direct exposure of the InGaZnO film to acidic pH solutions raises concerns over long term reliability, thus necessitating the investigation of a top-gate dielectric material and corresponding deposition method capable of providing both passivation and sensing functionality.
Passivation of the exposed semiconductor surface in bottom-gate InGaZnO TFTs has been shown to improve bias and illumination stress stability for electronic applications. The most straight-forward and reliable passivation strategies have focused on the use of organic dielectric materials [16–18]. However, these films are both low-k and thick (often several micrometers), thus rendering them unsuitable for high-sensitivity DG-TFTs. Inorganic thin films, such as SiO2 [19, 20] and Al2O3 [21], have also been studied, however it has been found that, depending on the deposition method, unintentional doping and plasma damage can have major effects on the passivated TFT's performance. There remains a need, therefore, to better understand and consequently control the deposition of passivation thin films on InGaZnO channels for high-performance TFTs.
In this paper, we focus on the use of TiOx as a passivation layer. TiOx has emerged as an attractive moisture barrier film [22] that has been employed to stabilize organic TFTs [23], and decelerate corrosion in photoelectrochemical cells [24]. As a result, TiOx has the potential to improve the stability and reliability of InGaZnO TFTs operated in liquid environments. Moreover, an initial study of low-temperature, TiOx-passivated InGaZnO DG-TFTs with high sensitivity gives further credence to its potential for sensing applications [25]. Additional reports, however, of InGaZnO TFTs passivated with oxidized Ti or sputtered TiOx have been conflicting thus far, with inconsistencies regarding how VTH or the response to bias stress change [26, 27]. Additionally, the performance dependence on the deposition temperature of TiOx passivation has not been systematically studied.
This work explores the use of atomic layer deposition (ALD) for the formation of a TiOx top-gate dielectric for sensing with and passivation of InGaZnO ISFETs. The high quality and low pin-hole density of ALD films makes this technique an ideal method for barrier film formation. Moreover, the ability to deposit the TiOx via ALD at low temperatures reduces the thermal budget and renders the process compatible with flexible or glass substrates that can be used for both display and wearable sensing applications. This paper presents the first analytical study of ALD temperature-dependent TiOx passivation effects in InGaZnO TFTs. It is demonstrated that low-temperature TiOx deposition on InGaZnO improves the bias stress stability of the TFTs and offers extended lifetimes of operation in acidic liquids. Moreover, it is shown that ALD TiOx provides near-Nernstian sensitivity. Altogether, this work paves the way for low-temperature and high-sensitivity ISFETs for chemical and bio-sensing applications.
2. Experimental
2.1. Device fabrication
To fabricate the TFTs depicted in figure 1, a 25 nm thick Al2O3 film was first deposited at 250 °C via ALD on a p++ silicon (Si) wafer that also serves as the bottom gate contact. The Al2O3 deposition used trimethylaluminum and water vapor as the aluminum and oxygen source, respectively, yielding a growth rate of 0.9 Å/cycle. A 50 nm thick InGaZnO (In:Ga:Zn = 1:1:5) semiconducting film was then deposited by pulsed laser deposition at room temperature and 25 mTorr O2 using a KrF excimer laser operating at 30 Hz and a 1.8 J cm−2 energy density. A mesa etch followed to isolate neighboring devices. Subsequently, 25 nm/200 nm thick chrome/gold (Cr/Au) source and drain contacts were formed using electron-beam evaporation and lift-off. The devices consisted of four parallel channels, each with a width (W) of 25 μm and length (L) of 10 μm (see insert in figure 1), thus representing an effective W:L ratio of 10:1. TFTs in this state constitute the bare reference device. Passivation of the devices was accomplished using a two-step process. Initially, the devices were subjected to a N2O plasma treatment at 50 kHz and 50 W RF power for 2 min. In previous work, this has been found to reduce the effect of hydrogen doping after PECVD SiO2 passivation steps [20]. The samples in the form of individual chips were then transferred to a Cambridge NanoTech Fiji ALD tool, in which 15 nm thick films of TiOx were deposited at four temperatures (100 °C, 150 °C, 200 °C and 250 °C). The precursors for TiOx deposition were water vapor and tetrakis(dimethylamino)titanium. The pulse times for these were 60 ms and 200 ms, respectively, while the purge times were adjusted according to the deposition temperature, ranging from 90 s at 100 °C to 10 s at 250 °C. Following passivation, the TFTs were annealed in an O2 atmosphere at 300 °C for 30 min.
Figure 1. Cross-sectional representation and micrograph of the InGaZnO double-gated ISFETs.
Download figure:
Standard image High-resolution image2.2. Microfluidic packaging for chemical sensing
In order to conduct tests in liquid, a microfluidic packaging process was also established. Firstly, a 6 μm thick SU-8 film (SU8-3005) was spun and patterned such that only the TiOx sensing regions are exposed to the liquid. Thereafter, a PDMS structure with patterned microfluidic channels was attached to the die for liquid analyte delivery. Figure 2 shows a picture of the die after the microfluidic packaging step, where a PMMA clamp has been used to bond the structure together. A motorized syringe pump was used to controllably flow the liquid solutions into the microfluidic cell where the TFTs are located. An off-chip and commercial flow-through Ag/AgCl reference electrode from Microelectrodes, Inc. was used.
Figure 2. Microfluidic packaging for InGaZnO DG-TFT (a) exploded model view of the entire microfluidic cell (b) photograph of fully packaged TFT within the microfluidic cell.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Electrical characterization
Figure 3(a) presents a comparison of the deposition-temperature-dependent TFT transfer characteristics (ID−VGS) in the linear mode of operation (VDS = 0.1 V) measured directly after the TiOx passivation step (prior to O2 annealing step). The threshold voltage (VTH) is defined as the value of VGS where ID = 1 nA. The on/off ratio (ION/IOFF) is calculated by evaluating the ratio of the maximum on-state current to the minimum off-state current. For the bare device, VTH = 1.3 V, while the on/off ratio (ION/IOFF) is 2 × 108. It is observed that, in all cases, passivation causes IOFF to increase, and VTH to shift negative. Moreover, the degree to which these changes occur depends on the deposition temperature. The 100 °C deposition increases IOFF to 10−8 A, while at 250 °C it reaches 10−4 A. Figure 3(b) depicts the absolute value of IG versus VGS behavior at this stage of fabrication. The bare device functions well with ∣IG∣ ∼ pA, but ∣IG∣ is found to increase significantly with increased passivation deposition temperature.
Figure 3. Comparison of (a) transfer characteristics (ID−VGS) and (b) magnitude of gate current (IG−VGS) immediately following passivation; comparison of (c) transfer characteristics and (d) field-effect mobility (μFE) following the annealing step in O2 at 300 °C after passivation. The temperatures in the legends correspond to the temperatures of ALD TiOx deposition. (VDS = 0.1 V).
Download figure:
Standard image High-resolution imageEach of the samples was then subjected to an O2 anneal for 30 min at 300 °C. After annealing, the gate current (not shown) decreased to under 100 pA in all of the devices. As shown in figure 3(c), the transfer characteristic also improved, with ION/IOFF increasing to 107 or more. Nonetheless, there remains a performance dependence on the passivation deposition temperature: as before, VTH shifts more negative as the temperature increases. It was found that the sub-threshold swing (S) tends to increase with increased deposition temperature. The bare device possesses an excellent S of 140 mV dec−1. The device passivated at 100 °C displays a slightly worse value of 185 mV dec−1, while the device passivated at 250 °C increases notably to 363 mV dec−1. These trends indicate that higher passivation temperature increases the trap density either in the InGaZnO bulk or the InGaZnO top surface where the TiOx is introduced [28]. Passivation clearly has a temperature-related effect on the field-effect mobility (μFE,max) too, as shown in figure 3(d). Though the bare device's maximum mobility was 16.5 cm2 V−1 s−1, the passivated devices all possess significantly reduced values. At 100 °C, μFE,max falls to 2.9 cm2 V−1 s−1 and increases with temperature to 6.5 cm2 V−1 s−1 at 250 °C. Table 1 summarizes the performance parameters with respect to passivation film deposition temperature. The source of these effects is further investigated in section 3.3.
Table 1. Summary of device characteristics as a function of ALD TiOx passivation film deposition temperature.
| Property | Bare | 100 °C | 150 °C | 200 °C | 250 °C |
|---|---|---|---|---|---|
| VTH (V) | 1.3 | 1.1 | 0.9 | 0.7 | 0.2 |
| S (mV dec−1) | 140 | 185 | 225 | 170 | 365 |
| μFE,max (cm2 V−1 s−1) | 16.5 | 2.9 | 3 | 3.4 | 6.5 |
| ION/IOFF | 2 × 108 | 2 × 108 | 1 × 107 | 9 × 107 | 5 × 108 |
3.2. Bias stress improvement
Positive bias stress (PBS) tests were conducted on the bare, 100 °C and 250 °C samples to contrast device stability. Each device was subjected to a forward bias condition of VGS = 5 V and VDS = 0.1 V for 1 h, as shown in figure 4. The bare device experienced a +1.1 V shift in VTH. In comparison, VTH of the 100 °C passivated device shifted by 0.2 V. Lastly, the 250 °C passivated device was observed to be the most stable of the devices, showing a nearly negligible 0.05 V positive shift. These results support previous reports [29] showing that the device stability is not solely dependent on the bottom dielectric (Al2O3)—semiconductor (InGaZnO) interface, but is also highly dependent on the electric-field induced adsorption of oxygen and desorption of water. Consequently, addition of a TiOx top-gate dielectric successfully suppresses this source of bias instability, and reduces the shift in VTH. The sub-threshold swing S was also affected differently following bias stress depending on the temperature of the passivation deposition. S in the bare device increased from 140 to 216 mV dec−1, while in the 100 °C device it only increased from 185 to 190 mV dec−1, and in the 250 °C device it improved from 363 mV dec−1 to 295 mV dec−1. While TiOx passivation clearly improves the bias stress stability of InGaZnO TFTs, the specific deposition temperature of the passivation film seems to only have a minor impact on these improvements.
Figure 4. Positive bias stress (PBS) results for the bare, 100 °C and 250 °C ALD TiOx passivated devices.
Download figure:
Standard image High-resolution image3.3. Material characterization
To better understand the above results, time-of-flight SIMS (TOF-SIMS) was carried out. Past studies using this technique have shown that high temperature ICP-CVD SiOx passivation can cause diffusion of In, Ga and Zn into the SiOx passivation layer, in turn reducing μFE and increasing S [30]. Furthermore, it has been reported that differences in the type of precursor (O2 versus H2O vapor) used for plasma-enhanced versus thermal ALD Al2O3 passivation, respectively, of InGaZnO can influence hydrogen doping, which subsequently determines the VTH shift [31]. Our SIMS analysis (figure 5), however, shows negligible differences in interdiffusion or hydrogen doping due to temperature effects. The latter also serves to support the use of the N2O plasma treatment prior to TiOx deposition.
Figure 5. TOF-SIMS depth profile for the TiOx passivated samples at (a) 100 °C and (b) 250 °C.
Download figure:
Standard image High-resolution imageIt is instead proposed that oxygen gettering by the TiOx passivation film is responsible for the measured characteristics, which has been observed in organic [23] and ZnO TFTs [32]. In both InGaZnO and InZnO films, oxygen vacancies act as electron donors, while the use of Ga as an oxygen getter enables superior mobility and VTH stability in InGaZnO [33]. Yen et al reported that e-beam evaporated TiOx films on InGaZnO111 and InZnO11 prior to S/D formation compete for oxygen [34]. Though both TFTs experienced negative shifts in VTH, S improved for the InGaZnO111 TFTs, but deteriorated in the InZnO11 TFTs. This difference was explained by the absence of Ga in InZnO.
Our results indicate that the strength of oxygen gettering is influenced by the TiOx deposition temperature, with higher temperatures generating more oxygen vacancies to reduce the VTH. The increase in S with passivation film deposition temperature can likewise be explained by the generation of traps from this phenomenon. Moreover, our results further elucidate the role of the InGaZnO composition in determining the compatibility of passivation strategies. Whereas Yen et al used a 1:1:1 target, our work used a 1:1:5 composition that is more akin to Ga-absent InZnO. Therefore, care must be taken in choosing the composition of InGaZnO, as well as its thin film deposition conditions, when selecting a passivation material and deposition process. The reduction in mobility post TiOx deposition is attributed to the generation of defects that are symptomatic of the increase in S and can increase the contribution of Coulomb scattering. This effect has been previously reported in TiOx-InGaZnO TFTs by evaluating the operation temperature-dependent behavior of such devices [35].
3.4. pH sensitivity of ALD TiOx
The above results underline the promise of ALD TiOx passivation of InGaZnO TFTs, in particular in terms of enhancing stability for electronic applications that require a dependable baseline. To subsequently validate the suitability of ALD TiOx as a top-gate dielectric in InGaZnO ISFETs for potentiometric chemical sensing, a 15 nm thick TiOx film was deposited via ALD at 150 °C on metal electrodes and its pH response was evaluated, as demonstrated in our previous work [36]. This method ties the Ag/AgCl reference electrode to ground (VREF = 0 V) as is standard for DG-TFT performance, and permits time-based measurements to be performed without the need of an external CHEMFET read-out circuit (e.g., source-follower). Both ISFETs and DG-TFTs are fundamentally limited by the behavior of the top gate dielectric-liquid interface, as demonstrated in the following [6]:
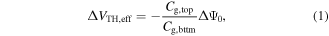
where Cg,top and Cg,bttm are the top and bottom oxide capacitances, respectively, and Ψ0 represents the top gate-electrolyte interface potential, given by [37]:
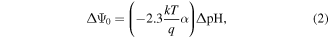
where k is the Boltzman constant, T is temperature, q is the charge of a single electron, α is a constant that depends on the quality of the dielectric-analyte interface and ΔpH is the change in pH. At a fixed temperature, therefore, the fundamental sensitivity of an ISFET (ΔΨ0/ΔpH) is dependent on the interface between the top gate dielectric under investigation and the liquid. In a DG-TFT, this fundamental sensitivity is amplified by the capacitance ratio Cg,top/Cg,bttm (which equals 1 in the case of the ISFET).
Figure 6(a) shows the transient response of ALD TiOx to buffer solutions with pH = 3, 5 and 7, which confirms the rapid and repeatable change in potential as a function of pH. The data has been normalized with respect to the initial voltage at t = 0 (pH = 7), and baseline drift has been corrected for. The hysteresis, which was calculated as the difference in potential between the two readings at pH = 5, is 10 mV. From the slope in figure 6(b), it is found that the sensitivity of ALD TiOx is 57.7 mV pH−1 and is therefore very close to the Nernstian Limit (∼59 mV pH−1). Additionally, the linearity of the response is 99.89%. These results are in line with past investigations of TiOx as a pH sensing layer using sol-gel formation [38] and RF sputtering [8], which recorded 50 mV pH−1 and 59.2 mV pH−1 respectively. The high sensitivity, high linearity and high dielectric permittivity of TiOx [8, 39] make it a highly attractive candidate as a top-gate dielectric/potentiometric sensing layer in InGaZnO ISFETs and/or DG-TFTs.
Figure 6. (a) Transient potentiometric response and (b) sensitivity of ALD TiOx thin film deposited at 150 °C to solutions of varying pH.
Download figure:
Standard image High-resolution image3.5. Stability in fluid for sensor deployment
In addition to sensitivity, it is important to evaluate the stability of InGaZnO ISFETs with an ALD TiOx top-gate dielectric. From our own tests, we have observed that a 50 nm thick InGaZnO115 film is completely etched in acid within 10 min, even when using a 1:150 dilution in water. Such short lifetimes would render these devices impractical for use in the field. To confirm that InGaZnO with a top ALD TiOx dielectric is suitably protected, the VGS−ID characteristic of a device submerged in pH = 5 buffer solution was periodically collected over a 24 h period (figure 7(a)). In between these measurements, the device was unbiased. The TFT continues to operate throughout the entire test, with very little VTH shift observed. As time passes, the OFF current decreases, thus improving the on/off ratio, but having no effect on the sensor's drift. The sensing baseline drift was monitored by reading out VGS for a reference drain current (IREF) of 10 nA, with an average drift of ≤1 mV h−1. These results clearly demonstrate not only that the InGaZnO thin film is protected from the acidic liquid environment, but that the DG-TFT's electrical behavior can also be maintained over an extended period of time. Previous studies confirming the high performance of ALD TiOx as a barrier layer are therefore consistent with our findings [22, 40].
Figure 7. 24 h evaluation of InGaZnO ISFET with ALD TiOx top-gate date dielectric stability in liquid environment (pH = 5): (a) periodic transfer characteristics with VDS = 0.5 V, (b) gate-source voltage (VGS) drift at IREF = 10 nA.
Download figure:
Standard image High-resolution image4. Conclusion
The fabrication of a high-performance InGaZnO ISFET and/or DG-TFT requires the carefully controlled deposition of a top-gate dielectric. This work reveals that the deposition temperature of ALD TiOx as the top-dielectric and passivation layer of InGaZnO115 TFTs plays a critical role in determining the performance of the resulting device. As the deposition temperature increases, VTH shifts downward and S increases. All of the TiOx passivated devices were found to have reduced μFE,max compared to the bare reference, while higher deposition temperatures offered the highest mobility. Moreover, PBS induced threshold voltage shift was reduced following passivation, resulting in more stable performance that is critical for reliable sensor deployment. These results are explained by the formation of oxygen vacancies in the InGaZnO film due to oxygen gettering, and point to the material composition's importance in determining a TFT's sensitivity to passivation material and process conditions. ALD TiOx deposited at 150 °C has moreover been demonstrated to possess a near-Nernstian pH response (57.7 mV pH−1) across the pH range of 3–7 that was investigated, and enables InGaZnO ISFETs to operate in liquid for >24 h with minimal drift. Future tests can be conducted to evaluate a wider range of pH values, as well as more complex biofluids that would be relevant to wearable (bio)chemical sensor applications. These results show the promise of TiOx/InGaZnO ISFETs fabricated at low temperatures for mobile chemical sensors.
Acknowledgments
This work was performed in part at the Georgia Tech Institute for Electronics and Nanotechnology (IEN), a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (Grant ECCS-1542174). S Pavlidis was supported by the Student Research Participation Program at US AFRL, 711th Human Performance Wing, Human Effectiveness Directorate, and administered by the Oak Ridge Institute for Science and Education (ORISE) though an interagency agreement between the US DOE and USAFRL. S Pavlidis and O Brand would like to acknowledge Dr Joshua A Hagen and Dr Nancy Kelley-Loughnane at US AFRL, 711th Human Performance Wing, Human Effectiveness Directorate for their helpful discussions and support.









