Abstract
The mechanical properties of three-dimensional self-assembled nanocrystals, as so-called supracrystals, are correlated with: i) the type of terminate groups of coating agents used to stabilize the nanocrystals; ii) their interactions with the nanocrystal surface atoms; iii) the nanocrystal morphology; iv) the interparticle distance between nanocrystals; v) translational and orientational ordering of atomic lattice planes of nanocrystals. The Young moduli evolve from a few MPa to GPa by controlling the various factors mentioned above.
Export citation and abstract BibTeX RIS
Introduction
Advances in the synthesis of colloidal nanocrystals (NCs) permit nowadays to exercise a fine control over their chemical composition, size and shape [1–3]. This makes possible to self-assemble quasi-spherical [4] NCs, with narrow-size distribution, into 3D crystalline structures called supracrystals (SCs). Spherical nanoparticles (NPs), assimilated to hard spheres with short-range interactions, self-assemble in various crystalline structures as face-centered cubic (fcc), body-centered cubic (bcc) and hexagonal close packing (hcp) lattices. The crystalline structure of such assemblies markedly depends on several factors such as vapor pressure, solvent, coating agents, inclusion of organic molecules (free coating agents, impurities, surfactant) etc.
Two growth mechanisms can be generated [4–6]: i) Heterogeneous growth: SCs films (layer-by-layer growth process) are formed by a slow solvent evaporation process of a NCs colloidal solution or at the air-toluene interface, by keeping the colloidal solution under solvent vapor saturation during several days. The latter SCs are called interfacial SCs. ii) Homogeneous growth: Shaped SCs grow in solution. Regular shapes such as octahedrons, triangles, hexagons or fivefold symmetric stars, characterize them.
SCs constitute ideal systems for studying novel chemical and physical properties originating from the collective interactions between NPs. Such a new class of materials exhibits specific properties that are neither those of the isolated NPs nor those of the corresponding bulk phase [7–14]. During the last two decades, a rather large variety of collective properties, due to dipolar interactions, have been investigated among which are collective optical and magnetic properties [8–11]. It has been shown that an intrinsic crystalline growth process takes place by mild annealing of small SCs, leading to the formation of triangular single fcc crystals [12]. Very surprisingly, properties fully analogous to those of atomic NCs are observed with SCs: Atoms are replaced by (incompressible) NCs and atomic bonds by coating agents (carbon chains), which act as mechanical springs holding the NCs together. This was demonstrated in various scientific domains:
- i)Crystal growth: SCs grown in solution show, at mesoscopic scale, similar shapes as observed at the nanoscale. Hence, the morphologies of NCs, SCs and minerals are similar at various scales from nanometer to millimeter [7].
- ii)One observes coherent breathing modes of atoms in NCs as well as of NCs in SCs [13].
- iii)As in atomic crystals, in SCs longitudinal acoustic phonons propagate with the speed of sound through coherent movements of NCs out of their equilibrium positions [14].
These intrinsic properties open up, from both technological and fundamental standpoints, a new research area, whereas it currently suffers of an extensive lack of knowledge.
Here our scope is limited to the elastic properties of SCs differing by the material (Au, Ag, Co), the growth mechanism (homogeneous/heterogeneous), the coating agent, NCs crystalline structure called nanocrystallinity.
Mechanical properties of supracrystals
The elastic properties of SCs are measured by using an atomic force microscope (AFM) in the acoustic mode. In the case of elastic (i.e., reversible) deformations, the Derjaguin-Muller-Toporov (DMT) model was used to calculate Young's modulus [15]. On the other hand, in the case of elasto-plastic (i.e., irreversible) deformations, the Oliver and Pharr model [16,17] is used to calculate Young's modulus of SCs (film or shaped) deposited on a silicon substrate from the following equation:

where Er is the "reduced modulus", A is the projected area of elastic contact and S is the experimentally measured contact stiffness, which is the slope of the initial unloading curve.
As the interfacial film has limited thickness, the Oliver and Pharr model cannot be used [18]. Thus, a plate model [19] is used to calculate Young's modulus of interfacial supracrystals. The indentation of the AFM tip was modeled as a uniform load over a very small central circular area and the edge of the plate (the interfacial films) is simply supported. Then Young's modulus could be calculated from eq. (2):

in which E is Young's modulus, w is the total applied load (force), a is the radius of the plate, ν is Poisson's ratio, y is the displacement of the central point and t is the thickness of the plate.
The mean E value obtained from a rather large number of indentations at different locations for each SC sample.
NCs are coated with organic molecules such as dodecanthiol (C12H25S), dodecylamine (C12H25NH2), oleic acid (C18H34COOH) or oleyamine (C18H34NH2) to prevent coalescence and to keep their integrities. NCs are Au, Ag and Co NPs differing by their crystalline structure called nanocrystallinity (single domain (SD) or polycrystal (POLY). As already mentioned, the NCs, characterized by a narrow size distribution self-assemble in 3D crystalline structure called supracrystals (SCs).
In order to verify that the measured elastic properties of the SCs are independent of the indenter size, the standard AFM tip is changed to a colloidal probe (C-probe) mounted on a cantilever of calibrated stiffness [20].  curve measurements were performed in the elastic deformation range, using a
curve measurements were performed in the elastic deformation range, using a  -diameter glass sphere on the film SCs of 8 nm ε phase Co NCs (ε-Co8). Then, the DMT model fits well the experimental
-diameter glass sphere on the film SCs of 8 nm ε phase Co NCs (ε-Co8). Then, the DMT model fits well the experimental  curves using a
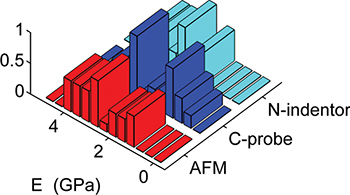
curves using a  -diameter sphere as an indenter. Figure 1 shows a rather good agreement between the various techniques.
-diameter sphere as an indenter. Figure 1 shows a rather good agreement between the various techniques.
Fig. 1: (Color online) Normalized distribution of E values of supracrystal films of ε-Co8 NCs deduced from different indenter geometries and testing methods. Red, blue and cyan colors indicate the distribution of E values obtained using AFM-based indentation with standard AFM tip, colloidal probe (C-probe) and nano-indentor (N-indentor), respectively.
Download figure:
Standard imageInfluence of the supracrystal growth processes
Let us consider Au SCs (films and shaped) of 5 nm SD NCs, with low size distribution  , produced through two simultaneous growth processes, described elsewhere [21]. In brief, by keeping the colloidal solution for at least 7 days in a saturated regime, a film appears at the air-toluene interface and simultaneously assembled precipitates are observed at the bottom of the beaker. Both (film and precipitated) are characterized by an fcc crystalline structure. The film is produced by formation of layer-by-layer SCs, which are floating at the air-toluene interface (fig. 2(a)). The precipitated SCs result from a homogeneous SC growth, which lie on the bottom of the beaker (fig. 2(b)). The ranging in size of the precipitated SCs, characterized by well-defined shapes, is
, produced through two simultaneous growth processes, described elsewhere [21]. In brief, by keeping the colloidal solution for at least 7 days in a saturated regime, a film appears at the air-toluene interface and simultaneously assembled precipitates are observed at the bottom of the beaker. Both (film and precipitated) are characterized by an fcc crystalline structure. The film is produced by formation of layer-by-layer SCs, which are floating at the air-toluene interface (fig. 2(a)). The precipitated SCs result from a homogeneous SC growth, which lie on the bottom of the beaker (fig. 2(b)). The ranging in size of the precipitated SCs, characterized by well-defined shapes, is  to
to  (fig. 2(b)). The precipitate surfaces show 2D nucleation without any dislocations.
(fig. 2(b)). The precipitate surfaces show 2D nucleation without any dislocations.
Fig. 2: (Color online) SEM images of the precipitated (a) and interfacial (b) 3D SCs. Inset: GISAXS patterns.
Download figure:
Standard imageThe high-resolution transmission electron microscopy, HRTEM (fig. 3(a)) and AFM images (fig. 3(b)) reveal the well-defined surface. The thickness of precipitated SCs (larger than  ) is more than 10 times larger [22] than the indentation depth (about 80 nm) making suitable the Oliver and Farr model to measure the Young modulus of shaped SCs (the substrate does not affect the measurements). The AFM images are taken before (fig. 3(d)) and after (fig. 3(e)) indentation. The average Young's modulus E is 5.1 ± 1.4 GPa. This E value is consistent with the elastic modulus in the GPa range and are related to the core material (e.g., PbS [23], CdSe [24], ... ). Note a slight difference between single-domain ε-Co (fig. 1) and Au NCs.
) is more than 10 times larger [22] than the indentation depth (about 80 nm) making suitable the Oliver and Farr model to measure the Young modulus of shaped SCs (the substrate does not affect the measurements). The AFM images are taken before (fig. 3(d)) and after (fig. 3(e)) indentation. The average Young's modulus E is 5.1 ± 1.4 GPa. This E value is consistent with the elastic modulus in the GPa range and are related to the core material (e.g., PbS [23], CdSe [24], ... ). Note a slight difference between single-domain ε-Co (fig. 1) and Au NCs.
Fig. 3: (Color online) (a) HRTEM image of precipitated SC surface. (b) High magnification of the AFM image showing different facets of precipitated SCs with highly ordered Au NCs. (c) Schematic illustration of nanoindentation measurements performed on precipitated SCs. (d) and (e): AFM images recorded before and after the indentation respectively. (f) HRTEM image of interfacial SC surface with dislocations. (g) High-resolution AFM image with dislocations. (h) Schematic illustration of nanoindentation measurements performed on free-standing interfacial SCs. (i) and (j): AFM image before and after indentation. The residual mark clearly shows the piles of NCs.
Download figure:
Standard imageThe interfacial films show highly ordered NCs with a long coherence length (more than a few micrometers (fig. 3(f)). The AFM image (fig. 3(g)) presents similar features as the HRTEM image, while straight edges are also observed as well as dislocations. Here, the Oliver and Farr model cannot be used because of the small film thickness (300 nm) and the indentation depths are typically around 80 nm, usual substrates (e.g., Si wafers). In practice, the interfacial SCs are deposited onto a transmission electron microscopy (TEM) grid with 2.6-mm-diameter circular holes and center-to-center distance of 4 mm (fig. 3(h)). The indentation is performed in the center of the free-standing films as illustrated in fig. 3(h). The AFM images are recorded before (fig. 3(i)) and after (fig. 3(j)) indentations. The residual mark is observed (fig. 3(j)) and NCs are piled up next to it indicating that the individual NCs are squeezed out to the surface during the indentation and the interactions between them are weak. Consequently it is expected a rather low stiffness of interfacial SCs. This is confirmed by the average value of the interfacial SC Young's modulus (240 ± 180 MPa). These differences between interfacial and precipitated SCs are very surprising, if we consider that they are produced by the same SD NCs both coated with C12H25SH and characterized by the same average distance between NCs  . The origin of such stiffness of interfacial SCs could be attributed to the layer-by-layer growing process, especially when reminding that the SCs with several GPa elastic moduli are formed in a totally different way [23].
. The origin of such stiffness of interfacial SCs could be attributed to the layer-by-layer growing process, especially when reminding that the SCs with several GPa elastic moduli are formed in a totally different way [23].
In former studies, NCs assemblies, i.e., monolayer [25,26] or shaped [23,24] SCs, are self-assembled on top of water or glass/ITO, respectively, which normally leads to holistic, robust structures as reported. Here, the interfacial SCs are formed at the air-solvent interface and the films are floating during the formation, which results in a soft layer-by-layer growth. These growing processes could lead to different mechanical properties. Podsiadlo and co-workers [23] also showed a difference in the mechanical behavior between evaporated NC films and shaped NC SCs where the film SCs have Young's moduli about several hundreds MPa. These values are close to the present ones and support the assumption from that the growth mechanism affects the mechanical properties. To verify the influence of the growth mechanism on the SCs elastic properties, let us dissolve in hexane the precipitated SCs characterized by 5.1 GPa as elastic modulus. The colloidal solution shows a well-defined plasmon resonance peak indicating that the NCs keep their integrity without coalescence. This solution is then slowly evaporated, forming layer-by-layer SCs films and Young's modulus is then measured. The average values of the elastic Young's modulus, deduced from the plate model is 550±160 MPa. This value, close to that of interfacial SCs (240±180 MPa), verifies the difference between the layer-by-layer films and the shaped SCs. The higher value of evaporated SCs could be due to the roughness and defects in the films that result from the evaporation mechanism. From these data it is concluded the SC growing processes tune their mechanical properties.
The junction between NCs
Young's modulus of bulk material (Au, Co and Ag) remains of the same order of magnitude (100 GPa < E < 220 GPa). These values are far from what was measured with SCs. The junction stiffness between NCs is much smaller than the elastic modulus of the NPs that can be considered as rigid. Hence under an applied force, the displacement localizes the strain in the junctions only. Young's moduli could be rationalized using an analogy between the periodic arrays of rigid spheres connected via compliant ligand junctions. From such assumption the strength of ligand-metallic atoms at the NC surfaces and ligand-ligand interactions play a key role in the elastic properties of the SCs. To support such claim let us consider the ligand-atoms at the NC surface and ligand-ligand interactions involved in the formation of supracrystals.
Ligand-atoms interactions: The groups currently used are thiol (SH), amine (NH2) and carboxylic (COOH) derivatives. The interactions between thiol ligand and noble metal are very strong (covalent). This is well experimentally demonstrated with Au NCs [27–29] and confirmed by atomistic simulation [30]. At the Au surface, significant damages and/or amorphous-like structures were observed [30]. With Ag atoms, the strength of the bonding remains strong but is less and not very well known. Furthermore, a chemical reaction between thiol ligand and Ag atoms takes place at the interface and Ag2S/AgO layer grows on the Ag surface. Such chemical reaction markedly modifies the NCs surface. The carboxylic group is covalently bound to the Co atoms [31]. With amine-terminated ligands (NH2), their binding on the metallic surfaces is very weak compared to SH and COOH groups [32]. NH2-terminated ligands do not produce almost any distortion in the crystalline structure of the metallic NPs [30]. This also explains the efficient ligand exchange process from amines to thiols, and not the other way around [33].
Ligand-ligand interaction: The saturated alkyl chains as coating agent (CnH2n+1) are well known to be characterized by a zig-zag molecular structure. At low solvent vapor pressure, strong ligand-ligand interactions take place tending to interdigitate. The calculated length of a zig-zag chain is similar to the measured interparticle distance,  , between NCs in SCs. This clearly indicates a "perfect" interdigitation between the alkyl chains. At high solvent vapor pressure, solvent and other molecules remain trapped between NCs and consequently induce an increase of the average
, between NCs in SCs. This clearly indicates a "perfect" interdigitation between the alkyl chains. At high solvent vapor pressure, solvent and other molecules remain trapped between NCs and consequently induce an increase of the average  . Oleyl groups are composed of two alkyl chains bound by a double bond. From a recent paper [34] the most likely structure is supported by the fact that the double bonds are polymerized through thermal treatment.
. Oleyl groups are composed of two alkyl chains bound by a double bond. From a recent paper [34] the most likely structure is supported by the fact that the double bonds are polymerized through thermal treatment.
The ligand-ligand and ligand-atoms interactions are the major parameters involved to control the elastic modulus. With zig-zag ligand molecules and strong ligand-atom interactions (Au-S-R), at zero solvent vapor pressure, the Young modulus increases with increasing chain length of the coating agent (table 1). At large solvent vapor pressure such dependency disappears. This trend is attributed to a change in the conformation of flexible ligands with the chain length and to free molecules trapped in the SC lattices [35]. With similar ligand and very weak ligand-atoms interactions (Ag-NH2), a marked drop in the Young modulus compared to others (table 1) is obtained. The best interdigitation process of surfactant molecules is obtained with long-tail surfactant molecules and large headgroup surface area [36]. Furthermore, the energy benefit is  [21] J by adding a -CH2- group into the alkyl chain [38]. This indicates that the best interdigitation process is obtained for a long-chain coating agent. By comparison SCs of amorphous Co NCs coated either with lauric acid (C12H25COOH) or with oleic acid (C18H33COOH), we could expect, for the same inter-particle distance, to observe a smaller Young modulus for NCs coated with C12 instead of C18. This is confirmed from experiments. The Young modulus of SCs of Co coated with C12 (80 ± 3 MPa) is much lower than that of Co NCs coated with C18 (700 ± 400 MPa).
[21] J by adding a -CH2- group into the alkyl chain [38]. This indicates that the best interdigitation process is obtained for a long-chain coating agent. By comparison SCs of amorphous Co NCs coated either with lauric acid (C12H25COOH) or with oleic acid (C18H33COOH), we could expect, for the same inter-particle distance, to observe a smaller Young modulus for NCs coated with C12 instead of C18. This is confirmed from experiments. The Young modulus of SCs of Co coated with C12 (80 ± 3 MPa) is much lower than that of Co NCs coated with C18 (700 ± 400 MPa).
Table 1:. A collection of results obtained with using the same technique of the material (Mat.), coating agent (CA): C12 zig-zag, C18 oleyl, diameter (D), interparticle distance (δpp), P solvent pressure vapor and Young's modulus (E). The crystalline structures of NPs presented here are either amorphous, polycrystalline or composed by a mixture of single domain and polycrystals.
| Mat. | CA | 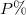 |
D (nm) | 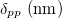 |
E (MPa) |
|---|---|---|---|---|---|
| Au [35] | C12H25-SH | 0% | 5 | 1.8 | 465 ± 125 |
| Au [35] | C12H25-SH | 39% | 5 | 3 | 385 ± 114 |
| Au [35] | C14H29-SH | 0% | 5 | 2.1 | 1538 ± 325 |
| Au [35] | C14H29-SH | 39% | 5 | 3 | 546 ± 192 |
| Au [35] | C12H25-SH | 0% | 7 | 1.9 | 63 ± 7 |
| Au [35] | C12H25-SH | 39% | 7 | 2.9 | 130 ± 60 |
| Ag [37] | C12H25-SH | 0% | 5.4 | 1.9 | 23.5 ± 18.5 |
| Ag [37] | C12H25-NH2 | 0% | 5.4 | 1.8 | 84.6 ± 18.5 |
| Ag [37] | C18H35-NH2 | 0% | 5.5 | 3.1 | 8.8 ± 2.4 |
| Ag [37] | C18-H35NH2 | 0% | 8 | 2.8 | 115 ± 28.6 |
| Co [36] | C18H34COOH | 0% | 6.9 | 3.4 | 700 ± 400 |
The marked drop of the Young modulus of Ag SCs compared to others (table 1) can be attributed to the weak interactions between surface atoms and ligands with a large flexibility of the ligands interacting through van der Waals forces.
Size effect of the building blocks (NCs) used to produce SCs
The influence of the NCs size on the mechanical properties remains an open question. The same experimental method used to deduce the SCs elastic modulus, differing by their coating agents and materials, gives rise to contradictions. Table 2 shows that the Young moduli of Au SCs decrease while those of Ag SCs increase and Co SCs remain constant with increasing NC size. Note that these data are highly reproducible. Furthermore Podsiadlo et al. [23] found an increase in Young's modulus with increasing PbS NCs size coated with oleic acid. These marked changes could be attributed to the ligand-ligand and ligand-atoms interactions. With Ag SCs we know that the ligand-atoms interactions are very weak. The SCs integrity is maintained by the ligand-ligand (oleyl-C18NH2) interactions. In such conditions, the van der Waals interactions between the cores increase on increasing the NCs and consequently the Young modulus also increases. This interpretation agrees with that of Podsiadlo et al. [23]. From simulations and experiments, the coverage of alkanethiol on Au NCs decreases on the increase of the NCs size [40–42]. The decrease of the ligands surface coverage may explain the drop of the mechanical properties mediated by ligand-ligand interactions of NPs assemblies. Furthermore, the drastic damages [30] due to interactions between thiol ligand and Au atoms could reduce the number of ligands attachment sites at the Au NCs surface. Consequently, the drop of the coverage could induce a drop in the E value on increasing the Au NCs size. With Co NCs, the perturbation due to the covalent bonding between Co atoms and the carboxylic group could be limited and the decrease in the coverage could be not large enough to perturb the elastic properties of Co SCs.
Table 2:.
A collection of results obtained by using the same technique of the material (Mat.), shape of SCs (SC), diameter (D), interparticle distance ( ) and Young's modulus (E). Except for 5 nm where the SCs are composed by single-domain Au NCs, at large NCs size the Au SCs are composed by a mixture of single domain and polycrystals. Ag NCs are characterized by an icosahedral structure. The crystalline structure of Co NCs is single domain ε phase. Au, Ag and Co NCs are coated with dodecanthiol (C12SH), oleylamine (C18NH2) and oleic acid (C18COOH), respectively.
) and Young's modulus (E). Except for 5 nm where the SCs are composed by single-domain Au NCs, at large NCs size the Au SCs are composed by a mixture of single domain and polycrystals. Ag NCs are characterized by an icosahedral structure. The crystalline structure of Co NCs is single domain ε phase. Au, Ag and Co NCs are coated with dodecanthiol (C12SH), oleylamine (C18NH2) and oleic acid (C18COOH), respectively.
| Mat. | SC | D (nm) |  |
E (MPa) |
|---|---|---|---|---|
| Au [39] | shape | 5.2 | 2.1 | 5100 ± 1400 |
| Au [39] | shape | 6.1 | 2.4 | 3200 ± 840 |
| Au [39] | shape | 7 | 2.6 | 1800 ± 550 |
| Au [39] | shape | 7.8 | 2.5 | 710 ± 380 |
| Au [39] | interface | 5.2 | 2.3 | 240 ± 180 |
| Au [39] | interface | 6.1 | 2.3 | 100 ± 60 |
| Au [39] | interface | 7 | 2.1 | 90 ± 50 |
| Au [39] | interface | 7.8 | 2 | 80 ± 40 |
| Ag [37] | film | 4 | 3.1 | 3.1 ± 0.5 |
| Ag [37] | film | 5.5 | 3.2 | 8.8 ± 2.4 |
| Ag [37] | film | 8 | 2.8 | 115 ± 28.7 |
| Co [20] | Film/shape | 7.1 | 3.3 | 1700 ± 500 |
| Co [20] | Film/shape | 8.2 | 3.2 | 2800 ± 900 |
| Co [20] | Film/shape | 9.0 | 3.3 | 2300 ± 900 |
Orientational and translational ordering of NCs in a SC
Facets due to their crystalline structures characterize the "quasi"-spherical NCs. This is well observed by high-resolution TEM on Au NCs. Figure 4 and the inset show the various Au NCs differing by their nanocrystallinities. The calculated facet surface indicates that single-domain (SD) Au NCs facet are larger than those polycrystalline counterparts [43].
Fig. 4: (Color online) High-resolution TEM (HRTEM) images of single domains (a), (b) and polycrystals (c), (d). NCs: cubooctahedral (a), truncated octahedral (b) icosahedral (c) and decahedral (d).
Download figure:
Standard imageLet us consider small fcc SCs (thin film of a few layers) of NCs building blocks differing by their nanocrystallinities (amorphous, polycrystals and SD NCs). High-angle electron diffraction (HAED) patterns exhibit several rings corresponding to electron diffraction by specific planes of atomic lattices with fcc crystalline structure in SCs. Note the presence of well-defined arcs in figs. 5(b) and (c), whereas they totally disappeared in fig. 5(a). Similar arcs in HAED patterns were previously observed in superlattices of self-assembled non-spherical building blocks [44], and especially consisting of Ag [45] and Au [46] NCs. They were explained in terms of NCs coherent alignment of the atomic lattice planes from one to another within the superlattice. Large facets in SD NCs compared to polycrystalline counterparts presumably favor such orientational ordering of the NCs atomic lattices and are expected to exhibit large enough area to induce their face-to-face orientation between neighboring NCs. This claim is supported by figs. 5(a) and (b): the HAED pattern of fcc SCs of polycrystalline 6 nm Au NCs (icosahedral and decahedral NCs) does not show any arcs (fig. 5(a)) whereas, with SD 5 nm Au NCs, the SCs exhibit arcs (fig. 5(b)). Au NCs differing by their nanocrystallinities are coated with dodecathiol (C12H23SH). The HAED pattern of SCs having icosahedral Ag NCs coated with oleylamine, C12H23NH2, (fig. 5(c)) shows well-defined arcs on the first, second and third rings indicating that the atomic lattice planes have a very long-range coherent alignment with those of neighboring NCs. The comparison of the various patterns shown in fig. 5 indicates a better alignment of the NCs atomic lattices with C12H23NH2 than with SD Au NCs coated with C12SH. This has to be related to the fact that weak binding takes place between Ag and C12H23NH2, whereas with the bonds of C12SH with Au NCs, create strong Au surface damages and consequently induces a decrease in the coherence length of the atomic alignment. With translational and orientational ordering of NCs in SCs we could expect to observe an increase in the SCs stiffness.
Fig. 5: (Color online) High-angle electron diffraction (HAED) patterns recorded from a thin film of close-packed (a) polycrystalline and (b) SD 5 nm Au NCs. (c) HAED pattern of 5.4 nm Ag NCs coated with oleylamine.
Download figure:
Standard imageTable 3 shows an increase of the Young moduli by comparing the values measured with SD SCs compared to their polycrystalline counterparts. This is valid for Au NCs coated with both hydrophobic and hydrophilic coating agents (C12-SH and H3N+-C12-SH) and with Co NCs coated with oleic acid (C18COOH). However, a careful comparison shows some slight changes:
- 1)
- 2)With Co NCs, the increase of the Young modulus is more pronounced with a highly compact structure as hcp NCs than with cubic phase. This was attributed to the fact that single-domain phase NCs are "quasi"-spherical NCs with less defined facet as observed with hcp Co NCs [49]. This clearly shows a hierarchy in the mechanical properties of SCs having SD NCs as building blocks.
- 3)With icosahedral Ag NCs we know from fig. 5(c) that the atomic lattice planes are aligned with a long-range coherence length. However, the Young modulus of SCs of such NCs is very weak. This has been attributed to the weak interactions between the coating agent head terminal group and the Ag surface atoms. However, the Young modulus is larger than that obtained with the same NCs having C12SH as coating agent. In the latter no arcs are observed in the HAED pattern and some chemical reactions with formation of Ag2S/AgO at the interface take place.
Table 3:.
A collection of results obtained by using the same technique of the material (Mat.), differing by their coating agents (CA), diameter (D), interparticle distance  and Young's modulus (E). With Au and Co we compared between single domain (SD) and polycrystalline phase (poly). Ag is characterized by icosahedral structure (ico). The coating agents are dodecanthiol (C12H25SH), N,N,N-trimethyl(11-mercaptoundecyl), ammonium chloride (+NH3-C12H24-SH), oleic acid (C18H35-COOH), oleyamine (C12H25-NH2).
and Young's modulus (E). With Au and Co we compared between single domain (SD) and polycrystalline phase (poly). Ag is characterized by icosahedral structure (ico). The coating agents are dodecanthiol (C12H25SH), N,N,N-trimethyl(11-mercaptoundecyl), ammonium chloride (+NH3-C12H24-SH), oleic acid (C18H35-COOH), oleyamine (C12H25-NH2).
| Mat. | CA | CS | D (nm) |  |
E (MPa) |
|---|---|---|---|---|---|
| Au [47] | C12H25SH | SD | 5.2 | 1.8 | 550 ± 160 |
| Au [47] | C12H25SH | Poly | 5 | 1.8 | 25 ± 9 |
| Au [48] | +NH3-C12-H24SH | SD | 7 | 1.8 | 315 ± 82 |
| Au [48] | +NH3-C12H24SH | Poly | 7.8 | 1.8 | 172 ± 39 |
| Co [49] | C18H35-COOH | SD (ε phase) | 7.1 | 3.3 | 1700 ± 500 |
| Co [49] | C18H35-COOH | SD (hcp) | 7.2 | 3.2 | 6600 ± 1500 |
| Co [49] | C12H25CO2H | A | 6.9 | 3.4.1 | 70090 ± 400 |
| Ag [37] | C12H25SH | ico | 5.4 | 1.9 | 23.5 ± 6.9 |
| Ag [37] | C12H25NH2 | ico | 5.4 | 1.9 | 85.61 ± 18.5 |
Conclusion and perspective
The data presented here clearly show that the stiffness of the SCs markedly depends on the material used, the coating agent and consequently the potential damage induced by the strong interactions between atoms at the NC surface and the terminal group of the coating agents and/or the weakness of these interactions, the average diameter of the building blocks used to produce SCs and nanocrystallinity.
According to the data presented here we need to have a better view on the influence of the NCs sizes on the stiffness of the material. The data presented, that are highly reproducible, need to be confirmed with other types of material. For future applications we need to be able to tune the stiffness of a material. For a given metal SCs, taking into account the data presented here we have a rather good opportunity to reach this goal. The tuning by three orders of magnitude of the Young moduli from large to weak stiffnesses seems to be possible if we choose accurately the coating agents: We have to take into account the strength of their interactions on the one hand between their terminal group with the NCs surface atoms and, on the other hand, between their alkyl chains: a large Young modulus, in GPa range, could be obtained by using a coating agent strongly bound to the NC interface and a non-solvent for the long alkyl chains to favor interdigitation process and homogeneous SC growth process. At the opposite end for very weak Young moduli, in the tenth range of MPa, very weak interactions between the surface atom and the coating agent as well as weak ligand-ligand interactions are produced by using a good solvent for the alkyl chains.
Acknowledgments
M-PP thanks Drs I. Arfaoui, A. Colak, M. Gauvin, S. Mourdikoudis C. Yan, Dr N. Yang and Dr Z. Yang who strongly contributed in the work presented here.
Footnotes
- (a)
Contribution to the Focus Issue Self-assemblies of Inorganic and Organic Nanomaterials edited by Marie-Paule Pileni.