Abstract
Thermocell, as a novel thermoelectric conversion device based on redox reaction or ion diffusion, has exhibited great advantages like high thermopower coefficient, cost effectiveness and good scalability for low-grade heat harvesting. Compared to the liquid-based thermocell, the gel-based version has garnered more and more attention because of its merits in leakage prevention, flexibility and integration, and significant achievements have been made in recent years. In this perspective, we briefly summarize the state-of-the-art progress on the gel-based thermocells. The basic working principle of thermocells is firstly introduced, followed by the discussion on the nuts and bolts for further system optimization. The emerging strategies on the performance improvement are highlighted as well as the underlying physics. Finally, the challenges and future directions in this field are prospected.
Export citation and abstract BibTeX RIS
Introduction
With the growing demand of energy, rising energy prices and environmental issues such as global warming, there is an urgent need for humans to find cleaner, more sustainable and more efficient energy sources [1–3]. However, more than 50% of the energy generated by various renewable or non-renewable energy sources is directly released into the environment in the form of low-grade heat with temperature less than 100 °C, including fossil combustion, industrial waste heat, automobile exhaust and human-body heat, etc. Coupled with the widespread distribution of geothermal energy, solar energy, and numerous natural resources that have not been fully exploited, a large amount of low-grade heat is wasted around the world by being released to the surrounding atmosphere at a rate of thousands of joules per second [4–8]. Therefore, capturing the widely distributed low-grade heat and converting it into available electrical energy are particularly important for alleviating the current global energy crisis.
Conventional thermoelectric conversion, first discovered in the 19th century, is based on electron-phonon transport and interactions of charge carriers in solid materials, which has been studied extensively over the past few decades [9–12]. However, suffering from the high cost of raw materials, lack of flexibility and low Seebeck coefficient on the order of several tens of  , these thermoelectric conversion devices, normally made of semiconductors, have great limitations in low-grade heat harvesting [13–16]. Alternately, thermoelectric conversion based on redox reaction or ion diffusion has attracted extensive attention in recent years. Due to its advantages such as high thermopower coefficient on the order of mV K
, these thermoelectric conversion devices, normally made of semiconductors, have great limitations in low-grade heat harvesting [13–16]. Alternately, thermoelectric conversion based on redox reaction or ion diffusion has attracted extensive attention in recent years. Due to its advantages such as high thermopower coefficient on the order of mV K , low cost and ease of scaling, it has shown a broad prospect in low-grade heat harvesting (see fig. 1) [17–20]. In the current researches, the thermocells can be classified into liquid-based or gel-based ones, which depends on whether the matrix is water or polymer gels. Considering the superiorities in leakproof performance, flexibility and integration, the gel-based thermocells show greater potential in practical applications, especially in the fields of utilizing human-body waste heat for powering the wearable electronics, soft temperature sensors, and self-powered electronic skins [21–24].
, low cost and ease of scaling, it has shown a broad prospect in low-grade heat harvesting (see fig. 1) [17–20]. In the current researches, the thermocells can be classified into liquid-based or gel-based ones, which depends on whether the matrix is water or polymer gels. Considering the superiorities in leakproof performance, flexibility and integration, the gel-based thermocells show greater potential in practical applications, especially in the fields of utilizing human-body waste heat for powering the wearable electronics, soft temperature sensors, and self-powered electronic skins [21–24].
Fig. 1: Low-grade heat sources and their potential utilization via gel-based thermocells.
Download figure:
Standard imageIn this perspective, we present a brief review of the gel-based thermocells proposed in the recent literature, including the working mechanisms, nuts and bolts, and the emerging strategies for performance improvement. Meanwhile, the remaining problems and challenges are pointed out, and some new insights are put forward to refuel the future exploring.
Working principles of gel-based thermocells
The energy harvesting from low-grade heat using gel-based thermocells can be attributed to two different mechanisms, namely the thermogalvanic effect (TG) and the thermodiffusion effect (TD). In this section, we will give an introduction of these two mechanisms.
Thermoelectric conversion using the thermogalvanic effect is mainly based on the temperature dependence of the redox reactions [25]. To illustrate this effect, consider a common redox reaction system as shown in fig. 2(a), which mainly consists of a pair of redox couple (ferro/ferricyanide [Fe(CN) /Fe(CN)
/Fe(CN) ]), electrolyte and two parallel electrodes. When a certain temperature gradient is applied to both ends of the electrodes, oxidation reaction and reduction reaction will occur at the hot end and the cold end, respectively, due to the different solvation entropy of the two redox ions. To be specific, the ferrocyanide (Fe(CN)
]), electrolyte and two parallel electrodes. When a certain temperature gradient is applied to both ends of the electrodes, oxidation reaction and reduction reaction will occur at the hot end and the cold end, respectively, due to the different solvation entropy of the two redox ions. To be specific, the ferrocyanide (Fe(CN) ) with smaller solvation entropy is oxidized into ferricyanide (Fe(CN)
) with smaller solvation entropy is oxidized into ferricyanide (Fe(CN) ) with higher solvation entropy by releasing an electron to the hot electrode, and the opposite process occurs simultaneously at the cold electrode. Due to the existence of ion diffusion and convection, the products at both ends will diffuse to the opposite electrode to continue the oxidation or reduction reaction. As a result, the system can produce a stable voltage output when an external load is attached. Analogous to the Seebeck coefficient of conventional solid thermoelectric materials, this electrochemical potential based on temperature-dependent redox reaction can be quantified using a thermogalvanic coefficient Stg
, expressed as follows:
) with higher solvation entropy by releasing an electron to the hot electrode, and the opposite process occurs simultaneously at the cold electrode. Due to the existence of ion diffusion and convection, the products at both ends will diffuse to the opposite electrode to continue the oxidation or reduction reaction. As a result, the system can produce a stable voltage output when an external load is attached. Analogous to the Seebeck coefficient of conventional solid thermoelectric materials, this electrochemical potential based on temperature-dependent redox reaction can be quantified using a thermogalvanic coefficient Stg
, expressed as follows:

where  and
and  are the potential difference and temperature difference between two parallel electrodes, SR
and SO
are the partial molar entropy of the reductant and oxidant, respectively, n is the number of electrons transferred in the redox reaction, and F is the Faraday's constant [26]. It is worth noting that the sign of Stg
is determined by the type of the redox couple, with positive and negative signs corresponding to p-type and n-type thermocells, respectively. For the redox system shown in fig. 2(a), the electric filed is in the opposite direction of the temperature gradient, which represents a typical p-type thermocell.
are the potential difference and temperature difference between two parallel electrodes, SR
and SO
are the partial molar entropy of the reductant and oxidant, respectively, n is the number of electrons transferred in the redox reaction, and F is the Faraday's constant [26]. It is worth noting that the sign of Stg
is determined by the type of the redox couple, with positive and negative signs corresponding to p-type and n-type thermocells, respectively. For the redox system shown in fig. 2(a), the electric filed is in the opposite direction of the temperature gradient, which represents a typical p-type thermocell.
Fig. 2: (a) Mechanism of the thermogalvanic effect. (b) Mechanism of the thermodiffusion effect.
Download figure:
Standard imageThe thermodiffusion effect, also known as the Soret effect [27], is the other mechanism of the gel-based thermocells, which is based on the ion immigration driven by a temperature gradient. Similar to the motion of electrons and holes in the Seebeck effect, the ion immigration also occurs in the electrolyte under the action of temperature difference. When the thermal diffusion coefficients of anions and cations are different, the overall electrical neutrality of the electrolyte will be broken, and different charge distributions will be formed at the hot and cold electrodes, resulting in the polarization of the electric double layer effect. At this point, if an external load is connected at both ends of the electrodes, the system will discharge outwards, forming a current in the circuit, and the electrolyte will gradually become electrically neutral. Note that unlike the continuous operation of the thermogalvanic effect, the thermodiffusion effect outputs electrical energy in a capacitive mode since there is no direct exchange of electrons between the electrolyte and the electrodes. Thus, a certain amount of time is needed after each discharge to carry out the next discharge process. Based on the Onsager transport theory, the conversion of heat to electricity can be described by a thermodiffusion coefficient Std , which is defined as

where q, n,  and D are quantity of electricity, ion concentration, Eastman entropy and diffusion coefficient, respectively, and the subscript i represents the ion species [28–29]. For the thermodiffusion system with KCl
and D are quantity of electricity, ion concentration, Eastman entropy and diffusion coefficient, respectively, and the subscript i represents the ion species [28–29]. For the thermodiffusion system with KCl  as a solute, as fig. 2(b) shown, the thermodiffusion coefficient can be simplified to
as a solute, as fig. 2(b) shown, the thermodiffusion coefficient can be simplified to

where e is elementary charge, and the subscripts  represent the cation and anion, respectively.
represent the cation and anion, respectively.
Nuts and bolts of gel-based thermocells
The nuts and bolts of gel-based thermocells mainly include ions, gel matrices and the electrodes, which will be shortly introduced as follows.
As the core part of the ionic thermoelectric devices, ions play the role of energy carriers, which indicates that the rational selection of them is of great significance. For gel-based thermocells based on the thermogalvanic effect, the thermopower coefficient Stg
depends on the entropy difference of the redox reaction, which is mainly determined by the inherent properties of the ions, including the ion structures and the ionic solvation shells. In addition, the acquisition of p- or n-type thermocells also relies on the species of redox ions, which can be explained by the difference in temperature sensitivity of different redox systems. In recent studies, the commonly used redox couple mainly consists of [Fe(CN) /Fe(CN)
/Fe(CN) ] [30–34], [Fe3+/Fe2+] [35–37], [Co(bpy)
] [30–34], [Fe3+/Fe2+] [35–37], [Co(bpy) /Co(bpy)
/Co(bpy) ] [38–40] and [I
] [38–40] and [I /I
/I ][41–43], etc. As for gel-based thermocells based on the thermodiffusion effect, the thermopower coefficient Std
arises from the difference in the thermal diffusion rate between anions and cations, which means a larger thermal diffusion rate corresponds to a larger thermopower coefficient. Generally, the thermal diffusion rate of ions is influenced by the ion size and the interaction between the ion and the solvent.
][41–43], etc. As for gel-based thermocells based on the thermodiffusion effect, the thermopower coefficient Std
arises from the difference in the thermal diffusion rate between anions and cations, which means a larger thermal diffusion rate corresponds to a larger thermopower coefficient. Generally, the thermal diffusion rate of ions is influenced by the ion size and the interaction between the ion and the solvent.
The main function of the gel matrices in the gel-based thermocells is to provide mechanical frameworks for the electrolyte systems, which makes the gel-based thermocells possess the advantage of leakage prevention when compared with the aqueous thermocells. However, the effect of the gel matrices on ion diffusion and the compatibility of the gel matrices with ions should be also taken into consideration. To date, various polymer gels such as gelatin [44], poly(vinyl alcohol) (PVA) [33, 37, 45], poly(sodium acrylate) [32, 46], poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) [38, 40], agar agar [46] and cellulose [47], have been explored by researchers from around the world. It is worth mentioning that the functional groups in the gel matrices may have influence on the performance of thermocells. For example, some charged functional groups could increase the difference in the thermal diffusion rate between anions and cations, in which case the thermopower coefficient would be enlarged. And polyanions with carboxyl functional groups, like carboxymethylcellulose [48] and poly(sodium acrylate) [46], promote the inclusion of higher concentrations of redox ions in the gel matrices.
Similar to ordinary cells, electrodes serve as the vital components of the gel-based thermocells, which have significant impacts on their output performance. In gel-based thermocells, the electrodes function as the reaction sites providers for ions and the mediums for electron transport. Considering the effect of charge transfer kinetics, metal electrodes such as platinum (Pt) [37], nickel (Ni) [18] and cooper (Cu) [44], have been widely used because of their performance in high catalytic activity, which means the overpotential of charge transfer process can be reduced. However, the high prices have become the biggest barriers to their large-scale applications. Alternatively, carbon-based electrodes have also attracted the attention of researchers due to their lower prices and porous surfaces [49].
Emerging strategies for performance improvement
To evaluate the output performance of thermocells, power factor (PF) and Carnot-relative efficiency  are defined as [30,50]
are defined as [30,50]

where Pmax
is the maximum power output,  is the temperature difference, S is the thermopower coefficient,
is the temperature difference, S is the thermopower coefficient,  is the effective electrical conductivity, A is the cross area, d is the inter-electrode distance, η is the practical efficiency of thermocells,
is the effective electrical conductivity, A is the cross area, d is the inter-electrode distance, η is the practical efficiency of thermocells,  is the Carnot cycle efficiency,
is the Carnot cycle efficiency,  is the effective thermal conductivity and Thot
is the temperature of the hot electrode.
is the effective thermal conductivity and Thot
is the temperature of the hot electrode.
Although the gel-based thermocells have shown great promise in low-grade heat harvesting, especially in body heat utilization, the performance improvement is essential to make them commercially viable. To this end, some emerging strategies have been proposed including the increase of thermopower coefficient, the enhancement of mechanical properties, the optimization of electrodes and the integration of systems, which will be discussed in the following section.
Increase of the thermopower coefficient
As mentioned above, the thermocells are mainly based on the thermogalvanic effect or thermodiffusion effect. For thermocells based on the thermogalvanic effect, the thermopower coefficient has been significantly increased by various means in recent years. For instance, Kim et al. achieved a doubling of the thermopower coefficient by adding an organic solvent to the electrolyte [51]. Duan et al. obtained a thermopower coefficient of  by introducing urea and guanidinium into the electrolyte containing 0.4M Fe(CN)
by introducing urea and guanidinium into the electrolyte containing 0.4M Fe(CN) /Fe(CN)
/Fe(CN) [52]. Most of these studies for improving the thermopower coefficient involved the rearrangement of the solvation shell caused by the additives, which resulted in the increase in the entropy change of the redox system. Nevertheless, the research objects of these strategies are mostly aqueous thermocells, and there is still a lack of research on enhancing the thermogalvanic effect in gel-based thermocells. Besides, the thermopower coefficient of gel-based thermocells using the thermogalvanic effect is lower than that in aqueous systems, which may be explained that the thermogalvanic effect is hindered to some extent in the gel matrices. For example, in the thermogalvanic thermocells with Fe(CN)
[52]. Most of these studies for improving the thermopower coefficient involved the rearrangement of the solvation shell caused by the additives, which resulted in the increase in the entropy change of the redox system. Nevertheless, the research objects of these strategies are mostly aqueous thermocells, and there is still a lack of research on enhancing the thermogalvanic effect in gel-based thermocells. Besides, the thermopower coefficient of gel-based thermocells using the thermogalvanic effect is lower than that in aqueous systems, which may be explained that the thermogalvanic effect is hindered to some extent in the gel matrices. For example, in the thermogalvanic thermocells with Fe(CN) / Fe(CN)
/ Fe(CN) as a redox couple, when poly(vinyl alcohol) and poly(sodium acrylate) were used as matrices, the thermopower coefficients of
as a redox couple, when poly(vinyl alcohol) and poly(sodium acrylate) were used as matrices, the thermopower coefficients of  and
and  were obtained by Yang et al. [53] and Wu et al. [46], respectively, which were a little lower than the value of about
were obtained by Yang et al. [53] and Wu et al. [46], respectively, which were a little lower than the value of about  for aqueous thermocells. Distinguished from the aqueous thermocells, the thermodiffusion effect can be observed more obviously in the gel matrices, and researchers have adopted some strategies to improve it. For example, Zhao et al. proposed an "ambipolar" polymer gel with a giant negative thermopower coefficient. They found that the dominating thermodiffused ions could be changed from anions to cations by adding neural PEG to polyfluorinated co-polymers, and a variable thermopower coefficient of
for aqueous thermocells. Distinguished from the aqueous thermocells, the thermodiffusion effect can be observed more obviously in the gel matrices, and researchers have adopted some strategies to improve it. For example, Zhao et al. proposed an "ambipolar" polymer gel with a giant negative thermopower coefficient. They found that the dominating thermodiffused ions could be changed from anions to cations by adding neural PEG to polyfluorinated co-polymers, and a variable thermopower coefficient of  to +14 mV K
to +14 mV K  could be obtained by tuning the composition, which is illustrated in fig. 3(a) [54]. In the studies mentioned above, researchers only focused on the enhancement of the thermogalvanic effect or the thermodiffusion effect, while few people paid attention to the combination of the two mechanisms. Under this background, a ternary system containing gelatin, potassium chloride (KCl) and Fe(CN)
could be obtained by tuning the composition, which is illustrated in fig. 3(a) [54]. In the studies mentioned above, researchers only focused on the enhancement of the thermogalvanic effect or the thermodiffusion effect, while few people paid attention to the combination of the two mechanisms. Under this background, a ternary system containing gelatin, potassium chloride (KCl) and Fe(CN) / Fe(CN)
/ Fe(CN) was proposed by Han et al. In the system shown as fig. 3(b), they demonstrated for the first time the synergistic effect of these two mechanisms, and a giant thermopower coefficient of +17 mV K
was proposed by Han et al. In the system shown as fig. 3(b), they demonstrated for the first time the synergistic effect of these two mechanisms, and a giant thermopower coefficient of +17 mV K  was obtained through the optimization of the composition [44].
was obtained through the optimization of the composition [44].
Fig. 3: (a) Schematic diagram of the interaction between ions, PEG molecules and polymer matrix [54]. (b) Schematic diagram of the diffusion, redox reaction and interaction of the ions in the ternary system [44]. (c), (d): output performance with different electrodes [44,49]. (e), (f): stretching and self-healing performance [55,56]. (g), (h), (i): different kinds of integration approaches.
Download figure:
Standard imageOptimization of electrodes
As is shown in eq. (4), the power factor (PF) of gel-based thermocells can be improved not only by increasing the thermopower coefficient (S), but also by enhancing the effective electrical conductivity  . Generally, a valid strategy for enhancing the
. Generally, a valid strategy for enhancing the  is to increase the ionic concentration. However, the high concentration of ions may increase the mass transport resistance and even lead to the decrease of the thermopower coefficient. Therefore, the optimization of electrodes becomes a better choice, which can improve the electrical conductivity
is to increase the ionic concentration. However, the high concentration of ions may increase the mass transport resistance and even lead to the decrease of the thermopower coefficient. Therefore, the optimization of electrodes becomes a better choice, which can improve the electrical conductivity  and the energy density without influencing other properties. As discussed above, the main function of the electrodes in gel-based thermocells is providing reaction sites for the ions and acting as the medium for electron transport. Thus, a key strategy for improving electrode performance is increasing the surface area. For example, a nearly fivefold increase in the energy density could be observed when gold coatings were added to the surfaces of the copper electrodes, as shown in fig. 3(c) [44]. Besides, the corrosion resistance of the electrodes could be improved, too. Except for the optimization of metal electrodes, some efforts have also been made to improve the carbon-based electrodes. For example, Liu et al. proposed a PEDOT/PSS-EFG/CNT composite carbon-based electrode by a simple drop-casting method [49]. It could be found that under the same conditions, the current density and power density of the composite electrodes were significantly higher than those of traditional platinum electrodes, as illustrated in fig. 3(d). The output performance of the electrodes was further improved when a carbon dioxide laser cutting was used to etch the microchannels on the electrode surfaces, which could be interpreted as the further increase of the electrode areas and the enhanced diffusion of redox species through the electrodes.
and the energy density without influencing other properties. As discussed above, the main function of the electrodes in gel-based thermocells is providing reaction sites for the ions and acting as the medium for electron transport. Thus, a key strategy for improving electrode performance is increasing the surface area. For example, a nearly fivefold increase in the energy density could be observed when gold coatings were added to the surfaces of the copper electrodes, as shown in fig. 3(c) [44]. Besides, the corrosion resistance of the electrodes could be improved, too. Except for the optimization of metal electrodes, some efforts have also been made to improve the carbon-based electrodes. For example, Liu et al. proposed a PEDOT/PSS-EFG/CNT composite carbon-based electrode by a simple drop-casting method [49]. It could be found that under the same conditions, the current density and power density of the composite electrodes were significantly higher than those of traditional platinum electrodes, as illustrated in fig. 3(d). The output performance of the electrodes was further improved when a carbon dioxide laser cutting was used to etch the microchannels on the electrode surfaces, which could be interpreted as the further increase of the electrode areas and the enhanced diffusion of redox species through the electrodes.
Enhancement of mechanical properties
Considering the various mechanical deformation that the gel-based thermocells may suffer in practical applications, such as bending, twisting and even accidental cutting, the enhancement of mechanical properties like toughness and stretchability also plays a significant role in improving the overall performance of gel-based thermocells. For example, inspired by the multi-network structures in biological tissues, Lei et al. proposed a double-network thermocell with extraordinary mechanical performance [55]. The double-network structure enhanced the energy dissipation while providing robust support for the matrices, resulting in a high stretchability of 217%, a large Young's modulus of 150 kPa and an excellent toughness of 2770 J m , which is remarkably higher than previously proposed thermocells, as shown in fig. 3(e). In order to further improve the durability and extend the lifetime of the gel-based thermocells, a ternary polymer hybrid consisting of a conjugated polymer (PANI), a non-conjugated anionic polyelectrolyte (PAAMPSA) and a physical crosslinker (PA) was designed by Akbar et al. [56]. On account of the dynamic hydrogen bonding and electrostatic interactions, the ternary system can not only withstand a stretch of 750%, but also maintain its thermoelectric properties after 30-cycle cutting and healing tests, as demonstrated in fig. 3(f).
, which is remarkably higher than previously proposed thermocells, as shown in fig. 3(e). In order to further improve the durability and extend the lifetime of the gel-based thermocells, a ternary polymer hybrid consisting of a conjugated polymer (PANI), a non-conjugated anionic polyelectrolyte (PAAMPSA) and a physical crosslinker (PA) was designed by Akbar et al. [56]. On account of the dynamic hydrogen bonding and electrostatic interactions, the ternary system can not only withstand a stretch of 750%, but also maintain its thermoelectric properties after 30-cycle cutting and healing tests, as demonstrated in fig. 3(f).
Integration of systems
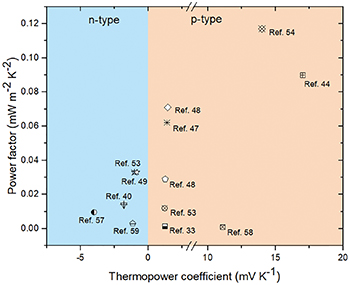
Due to the limitation of output performance of a single thermocell, device integration plays an important role for the applications of thermocells in practical scenarios. As shown in fig. 3, there are three kinds of integration approaches as reported in the literature: parallel connection using one type of thermocell (fig. 3(g)), series connection using one type of thermocell (fig. 3(h)) and series connection using two types of thermocells (p-type and n-type) (fig. 3(i)), respectively. As described in the working principle section, the type of a thermocell is defined by the direction of the temperature field and the electric field, which means the same direction represents a n-type thermocell and a p-type one otherwise. Zhou et al. proposed a parallel connection scheme using ferrous/iron ions (Fe2+/Fe3+) as redox couple [37]. In this parallel system, each thermocell releases an electron to the cold electrode and receives it at the hot electrode, thereby the overall performance will be enhanced after the collection. However, this integration method can only improve the current density of the thermocells, but has little effect on the increase of the output voltage. In order to achieve more efficient power output, the series connection using one type of thermocell has also been studied by many researchers. For example, by connecting 25 single cells in series, a considerable output voltage of 2.2 V was obtained when the temperature difference was about 10 K, which provides the possibility of practical application for the power supply of wearable electronic devices [44]. Although the series connection has better output performance, there are still some problems such as the more complex integration process, and the inevitable increase of the contact resistance between the wires and the electrodes. Similar with p-n combination in traditional thermoelectric devices, two types of thermocells can also be used for series integration. Using this integrated approach, an increase of output voltage from 20.1 to 351 mV could be observed with the number of p-n pairs increasing from 1 to 18, under a temperature difference of  10 K. Meanwhile, the internal electrical resistance would also increase with the increase of p-n pairs, so that the power output would reach a peak when the p-n pairs increased to a certain number [49]. It is worth mentioning that although the p-n combination simplifies the integration process and reduces the contact resistance, there is still room for a breakthrough in the output performance because of the lack of n-type thermocell with high performance so far, as shown in fig. 4.
10 K. Meanwhile, the internal electrical resistance would also increase with the increase of p-n pairs, so that the power output would reach a peak when the p-n pairs increased to a certain number [49]. It is worth mentioning that although the p-n combination simplifies the integration process and reduces the contact resistance, there is still room for a breakthrough in the output performance because of the lack of n-type thermocell with high performance so far, as shown in fig. 4.
Download figure:
Standard imageConcluding remarks
The utilization of low-grade heat has been a hard nut to crack during these years due to the limitation of thermoelectric conversion technology, but the emergence of ionic thermoelectric devices (thermocells) provide a new way to break this dilemma. Among them, the gel-based thermocells have shown greater potential in practical applications, especially in the utilization of body heat for powering the wearable electronics, soft temperature sensors and electrical skins. However, as a new technology, there are still some problems to be solved. Firstly, despite the high thermopower coefficient of the gel-based thermocells, their energy density and energy conversion efficiency still need to be further improved when compared with traditional solid thermoelectric materials. Toward this end, in addition to continuously increasing the thermopower coefficient, attempts such as how to enhance the effective electrical conductivity and reduce the effective thermal conductivity could also be taken into consideration. Secondly, the researches on the microscopic mechanisms of gel-based thermocells are not in depth enough, including the action mechanism of ions and electrodes, the synergistic and competitive mechanism between thermogalvanic effect and thermodiffusion effect, etc. The study of these mechanisms can be beneficial to the optimal design of the gel-based thermocells, so it is worthy of further exploration. Moreover, the basic elements of gel-based thermocells, such as redox couple, thermodiffusive ions, electrodes and gel matrices, still need further selection and design optimization to enhance the overall performance.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (51706079, 52076087), Wuhan City Science and Technology Program (2020010601012197).
Data availability statement: All data that support the findings of this study are included within the article (and any supplementary files).