Abstract
Since the International Commission on Radiological Protection recommended reducing the occupational equivalent dose limit for the lens of the eye in 2011, there have been extensive discussions in various countries. This paper reviews the current situation in radiation protection of the ocular lens and the discussions on the potential impact of the new lens dose limit in Japan. Topics include historical changes to the lens dose limit, the current situation with occupational lens exposures (e.g., in medical workers, nuclear workers, and Fukushima nuclear power plant workers) and measurements, and the current status of biological studies and epidemiological studies on radiation cataracts. Our focus is on the situation in Japan, but we believe such information sharing will be useful in many other countries.
Export citation and abstract BibTeX RIS
1. Introduction
In April 2011, the International Commission on Radiological Protection (ICRP) recommended lowering the occupational equivalent dose limit for the lens of the eye from 150 mSv/year to '20 mSv/year, averaged over defined periods of 5 years, with no single year exceeding 50 mSv' [1]. Since then, there have been extensive discussions all over the world. For instance, in Europe, Member States of European Union were required in December 2013 to implement a new dose limit, by February 2018, of '20 mSv in a single year or 100 mSv in any five consecutive years subject to a maximum of dose of 50 mSv in a single year' [2], which is consistent with the new ICRP dose limit. In the US, the Nuclear Regulatory Commission (NRC) started discussions to reduce the limit from 150 mSv/year to 50 mSv/year, but decided to keep it unchanged following one year of public consultation [3]. In contrast, the US National Council on Radiation Protection and Measurements (NCRP) initially considered keeping the dose limit unchanged at 150 mSv/year [4], but finally decided to reduce it to 50 mGy/year for low linear energy transfer (LET) radiation, and the absorbed dose is multiplied by the relative biological effectiveness (RBE) value yielding gray-equivalent (Gy-Eq) for high-LET radiation [5, 6]. In Canada, final discussions are continuing, to implement the dose limit of 50 mSv/year and 100 mSv over five years. Thus, the national decisions being made vary significantly.
In Japan, the Basic Subcommittee of the Radiation Council is discussing implementation of the new ICRP recommendations into national law. As a procedure, approval by the General Assembly of the Radiation Council is followed by revision of national laws. For the ICRP 1990 Recommendations [7], the Basic Subcommittee initiated discussion in February 1991, and submitted its interim report to the General Assembly in February 1998. Following approval by the General Assembly in August 1999, the ICRP 1990 Recommendations were implemented into national law in April 2001. For the ICRP 2007 Recommendations [8], the Basic Subcommittee initiated discussion in March 2008, and issued its second interim report in January 2011. The accident at Tokyo Electric Power Company (TEPCO)'s Fukushima Daiichi Nuclear Power Plant (1F-NPP) occurred in March 2011, and the Radiation Council was reorganized in September 2012, from being under the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to being under the Nuclear Regulation Authority (NRA). Since that reorganization, the Radiation Council has yet to resume discussion on implementation of the ICRP 2007 Recommendations. However, it is important for experts to be prepared for upcoming discussions. The Expert Committee was therefore established within the Japan Health Physics Society (JHPS) for two terms (the first term in 2013–15 and the second in 2015–17) to review current knowledge and status of radiation protection of the ocular lens and to discuss potential concerns as to implementation in Japan of the new occupational equivalent dose limit for the lens. This paper reviews the current situation and the series of discussions held by the JHPS Expert Committee. Our focus is on the situation in Japan, but we believe such information sharing will be useful to many other countries.
2. Occupational lens exposure in Japan
2.1. Methods of radiation monitoring and dose assessment
In the current Japanese regulations on radiation safety [9, 10], the effective dose limits for occupational exposure of workers are 100 mSv over five consecutive years and 50 mSv in any single year under normal operations. The equivalent dose limits for the skin and the lens of the eye are 500 and 150 mSv/year, respectively.
For individual monitoring, the effective dose and the equivalent dose to the skin are measured using operational quantities Hp(10) and Hp(0.07), respectively, except for neutron fields, in which only Hp(10) is exclusively measured. Hp(10) or Hp(0.07) measured, but not Hp(3), is used to estimate the equivalent dose to the lens of the eye (details given in section 3).
Specifically, personal dosemeters, such as a radiophotoluminescence dosemeter (RPLD) [11], optically stimulated luminescence dosemeter (OSLD) [12], and thermoluminescent dosemeter (TLD) [13], are usually worn on the chest or the waist by males and females, respectively. When a worker is partially shielded with a protective apron, additional dosemeters are worn at the neck level or at the location with the highest expected dose, such as at the fingertips (if necessary). In this case, the effective dose is estimated as 0.11 × Ha + 0.89 × Hb [14, 15], where Ha is the Hp(10) with the over-apron dosemeter at head or neck level and Hb is the Hp(10) with the under-apron dosemeter at chest or waist level, and the equivalent dose of the lens is estimated from the Hp(10) or Hp(0.07) with the over-apron dosemeter.
2.2. Dose distribution to radiation workers (except for nuclear workers)
The respective licensee manages the data for employees except for nuclear workers (e.g., medical, general industry, and research and education fields).
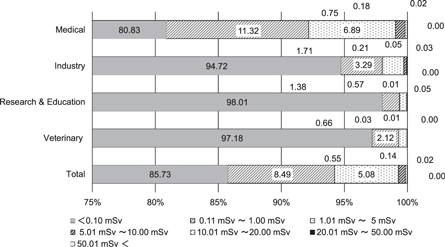
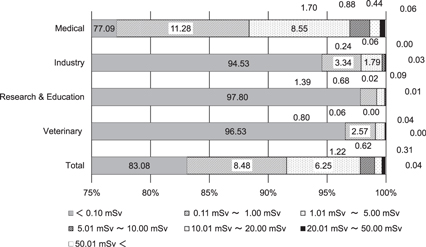
Figures 1 and 2 show the distribution of the effective dose and equivalent dose to the lens in radiation workers in fiscal year (FY) 2015 [16]. In Japan, the FY begins in April and ends in March of the following year. As such, the annual dose is the accumulated monthly dose from April to March. These data are provided by two personal dose monitoring services, except for nuclear fields [16, 17]. These are divided into several occupational categories. The dose is measured using RPLDs, provided that the personal dosimetry service companies are traceable to national standards. The annual number of workers using the services is about 500 000 [18]. The fraction of workers in each category does not differ between the monitoring service companies. The percentages of workers in the medical and other industrial fields (including non-destructive inspection) are 65% and 15% of the total workers, respectively. The percentages of workers in non-destructive inspection, educational and veterinary fields are about 1%, 13% and 3% of the total workers, respectively. The percentage of workers who received an effective dose of less than 0.1 mSv is about 80%–90% of the total workers. There are some workers receiving a high dose, as shown in the figures. The number of workers whose effective dose or equivalent dose to the lens exceeds 20 mSv/year is limited to a few persons in the non-destructive inspection field [16]. In the general medical field, the number of workers whose equivalent dose to the lens exceeds this value is about 1000 persons per year.
Figure 1. Distribution of effective dose for radiation workers (except for the nuclear industry) in FY 2015, presented by Chiyoda Technol Co [15].
Download figure:
Standard image High-resolution imageFigure 2. Distribution of equivalent dose to the lens of the eye by occupation in FY 2015, presented by Chiyoda Technol Co [16].
Download figure:
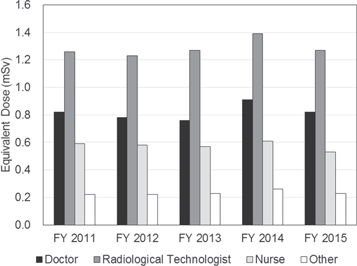
Standard image High-resolution imageFigure 3 shows the average equivalent dose to the lens for workers in each occupational category [15]. The average equivalent dose to the lens is the highest for workers in a medical field. The average dose for doctors, radiological technologists, nurses, and other health care workers is shown in figure 4. In the general medical field, the average equivalent dose is highest in radiological technologists (1.26 mSv/year in FY 2015).
Figure 3. Average equivalent dose to the lens of the eye by occupation, presented by Chiyoda Technol Co [16].
Download figure:
Standard image High-resolution imageFigure 4. Average equivalent dose to the lens of the eye in the medical field, presented by Chiyoda Technol Co [16].
Download figure:
Standard image High-resolution image2.3. Medical workers
2.3.1. Interventional radiology (IVR) and angiography
In 1994, the US Food and Drug Administration published Avoidance of Serious x-ray-Induced Skin Injuries to Patients during Fluoroscopically-Guided Procedures, in order to deal with skin injuries to patients owing to x-rays used in the medical field, in consideration of an increasing number of fluoroscopically-guided IVR procedures [19]. In Japan, in 1995, the Radiation Protection Committee of the Japanese Radiological Society took this issue into account in a statement regarding exposure of patients and physicians during IVR procedures [20].
In 2000, ICRP issued Publication 85 to reduce and protect against exposure of the skin and eye in patients and medical staff [21]. Moreover, in Japan, the relationship between dose and its potential impact on the skin and eye during fluoroscopically-guided procedures and cases of skin injuries in Japanese patients was described in 2004 [22], highlighting the importance of estimating the exposure of the skin of patients. In 2006, a Joint Working Group of the Japan Circulation Society (JCS) published the Guideline for Radiation Safety in Interventional Cardiology (IC) (revised in 2013) [23, 24]. These guidelines addressed general issues (e.g., basic information about radiation exposure and its health effects) and specific issues (e.g., dose response for skin injuries and strategies for reducing exposure in medical personnel and during the treatment of pregnant patients). Other specific issues include the necessity of protecting the eyes and the thyroid of medical staff by using safeguards, and the importance of standing position, time, and the choice of protective clothing.
In 2000, exposure of patients and operators was measured during the course of 39 transcatheter arterial embolization procedures at five medical facilities in Japan [25]. The average equivalent doses to the skin of the patients and to the skin between the eyebrows of the operators were 973 ± 681 mSv/procedure and 0.04 ± 0.04 mSv/procedure, respectively.
In 2003, TLDs and RPLDs were used to measure the skin surface doses for a physician during endovascular treatment for brain disease [26]. The mean surface skin doses at the left and right corners of the eye and the neck of the physician were 0.012 mGy/procedure, 0.102 mGy/procedure, and 0.015 mGy/procedure, respectively, under the device mode with an average tube voltage of 84.5 kV, an average tube current of 12.9 mA and a total exposure time of 15.5 min. The doses to the fingers and eye (between the eyebrows) were measured at four facilities using TLDs [27]. For procedures such as catheter placement, embolization treatment of a cerebral aneurysm (ETCA), and percutaneous transluminal coronary angioplasty (PTCA), the dose to the lens (Hp(0.07)) during PTCA procedures was the highest. The average dose and maximum dose during PTCA procedures were 0.3 mSv/procedure and 2.1 mSv/procedure, respectively.
A dose measurement system was developed where the skin surface dose distribution in a patient body is evaluated with more than 50 RPLDs [28, 29]. In that study, direct measurement of the patient dose as well as dose mapping could be performed easily where patients and operators had a vest and head cap to which RPLDs were attached. The average entrance skin doses at the left and right eye positions of the operator, taken over 25 therapeutic IVR procedures, were 0.254 ± 0.338 mGy and 0.028 ± 0.032 mGy, respectively, for a mean fluoroscopic time of 44.1 ± 31.2 min [28]. Recently, a study using the same method was performed on the diagnostic reference level and exposure for medical staff during IVR procedures for treating brain disease [30].
In 2014, a multicenter study was performed by the Japanese Society of Radiological Technology (JSRT) to evaluate the lens dose in medical staff, such as physicians and nurses, during non-vascular endoscopic IVR procedures at Japanese medical facilities [31, 32]. In that study, the medical staff wore protective glasses with small OSLDs, and the lens dose during procedures was measured for one month at 11 medical facilities [32]. At the 2015 autumn meeting of the JSRT, problems with the radiation management for the lens, correction coefficients estimated from measurements at the neck to the equivalent dose to the lens, and a survey on lens dose of operators during cerebral aneurysm embolization were reported [33–35].
There have been several studies on occupational exposure that have also conducted dose measurement during IVR procedures [36–38], where photon spectra were considered for more precise evaluation of the lens dose and for the standardization spectra for the test of dosemeters.
A performance of radiation protective eyewear used in the medical field was evaluated, where the dose reduction rates for 0.07 mmPb and 0.75 mmPb protective eyewear were measured using an over-tube type of fluoroscopy device [39]. Depending on the type of eyewear, the reduction rate of the left eye surface dose of a phantom ranged between 9% and 62% when the tube was located on the left side of the phantoms. These results indicate that the dose reduction rate of the lens dose by eyewear and the distance between the eyewear and the eye are important factors in the reduction of lens dose.
Other studies have been performed on the exposure of Japanese medical staff during computed tomography (CT) angiography procedures [36, 37]. The dose distribution of scattered radiation near the gantry was measured using OSLDs during multi-detector row CT (MDCT) procedures within the CT rooms. The maximum dose, Hp(10), at 50 cm from the gantry and 150 cm above the floor, was 1.47 mSv/examination [40]. The skin surface dose was measured using RPLDs during CT-guided biopsy procedures for lung cancer [41]. These annual equivalent doses to the skin of the fingers, with and without a protector (e.g., a needle guide) were less than 0.0047 mSv and 0.63 mSv, respectively. The annual equivalent doses to the lens measured at a position between the eyebrows, with and without the protector, were 0.025 mSv and 0.02 mSv, respectively. The equivalent dose to the lens was extremely low under CT-guided biopsy procedures [41]. Table 1 lists doses for medical staff and medical procedures.
Table 1. Dose to the medical staff for different procedures.
| Procedures | Staff | Measurement positions | Types of dosimeter | Dose | References | |
|---|---|---|---|---|---|---|
| Transcatheter arterial embolization | physicians | between eyebrows | skin dose | TLD | 0.04 ± 0.04 mGy/procedure | Ishiguchi T et al 2000 [25] |
| for hepatcocellular carcinoma | skin dose | 0.15 mGy (maximum) | ||||
| Endovascular treatment (Head) | physicians | right eye | skin dose | TLD, RPLD | 0.012 mGy/procedure | Nishizawa K et al 2003 [26] |
| left eye | skin dose | TLD, RPLD | 0.102 mGy/procedure | |||
| neck | skin dose | TLD, RPLD | 0.015 mGy/procedure | |||
| Percutaneous transluminal | physicians | between eyebrows | Hp(0.07) | TLD | 0.3 ± 0.45 mSv/procedure (average) | Yamagishi M et al 2003 [27] |
| coronary angioplasty | 2.1 mSv/procedure (maximum) | |||||
| Catheter placement | physicians | between eyebrows | Hp(0.07) | TLD | 0.13 ± 0.74 mSv/procedure (average) | |
| 0.35 mSv/procedure (maximum) | ||||||
| Embolization (Head) | physicians | between eyebrows | Hp(0.07) | TLD | 0.24 ± 0.27 mSv/procedure (average) | |
| 1.3 mSv/procedure (maximum) | ||||||
| Interventional radiology | operators | right eye | skin dose | RPLD | 0.254 ± 0.338 mGy/procedure | Moritake M et al 2008 [28] |
| (Arteriovenous fistula etc.) | left eye | skin dose | RPLD | 0.028 ± 0.032 mGy/procedure | ||
| Coronary artery angiography | physicians | left eye | Hp(3) | TLD | 0.10–0.15 mSv day−1 | Yokoyama S et al 2016 [36] |
| CT-angiography | operators | 50 cm from a gantry, | ||||
| 150 cm height | Hp(10) | OSLD | 1.47 mSv/procedure | Tomita H et al 2004 [40] | ||
| CT-angiography for lung | operators | finger | skin dose | RPLD | <0.0047 mSv/year (with a needle guide) | Takatsuka M et al 2005 [41] |
| 0.63 mSv/year (without a needle guide) | ||||||
| between eyebrow | skin dose | RPLD | 0.025 mSv/year (with a needle guide) | |||
| 0.02 mSv/year (without a needle guide) | ||||||
| PET imaging (18 F) | operator etc. | 20 cm from a tube | Hp(0.07) | calculation | 0.423 pSv/min.Bq | Katoh T et al 2011 [42] |
| operator etc. | 5 cm from a tube | Hp(3) | calculation | 0.070 mSv min−1 | Yokoyama S et al 2014 [43] | |
| doctors | chest or abdomen | Hp(10) | RPLD etc. | 0.025–0.040 mSv/month | Medical Science and Pharmaceutical Committee of Japan Radioisotopes Association 2008 [44] | |
| radiological technologists | chest or abdomen | Hp(10) | RPLD etc. | 0.11 mSv/month (average) | ||
| pharmacists | chest or abdomen | Hp(10) | RPLD etc. | 0.025 mSv/month (average) | ||
| cyclotron operators | chest or abdomen | Hp(10) | RPLD etc. | 0.10 mSv/month (average) | ||
| radiophamaceutical synthesis technicians | chest or abdomen | Hp(10) | RPLD etc. | 0.35 mSv/month (average) | ||
| nurses | chest or abdomen | Hp(10) | RPLD etc. | 0.10 mSv/month (average) | ||
| Brachytherapy using 125 I | doctors | — | Hp(10) | calculation | 0.0033 mSv/procedure | Medical Science and Pharmaceutical Committee of Japan Radioisotopes Association 2011 [45] |
| (13.1 MBq/seed × 80 seeds) | doctors | — | Hp(0.07) | calculation | 0.041 mSv/procedure | |
| nurses | — | Hp(10) | calculation | 0.0015 mSv/procedure | ||
| nurses | — | Hp(0.07) | calculation | 0.007 mSv/procedure |
2.3.2. Nuclear medicine diagnostics
Knowledge of exposure of the lens during nuclear medicine diagnostics has not yet been accumulated sufficiently to discuss the equivalent dose to the lens in Japan. There are calculated estimations for 18F-fluorodeoxyglucose (18F-FDG) used for a positron emission tomography (PET) at medical facilities [42, 43]. In those studies, it was estimated that the equivalent dose to the skin (Hp(0.07)), at 60 cm from an injection tube became 20% of the dose at 20 cm from the tube [42], and the calculated equivalent dose to the lens (Hp(3)) was 70 μSv/min at 5 cm from the tube [43]. In 2008, the Medical Science and Pharmaceutical Committee of the Japan Radioisotope Association (JRIA) prepared a questionnaire to determine the occupational dose at nuclear medical facilities [44]. The effective doses for doctors, radiological technologists, pharmacists, cyclotron operators, nuclear medicine technologists (who produce or synthesize nuclear medicine) and nurses were estimated from doses measured using RPLDs and electronic dosemeters at the facilities. Of these, radiological technologists received the highest exposure, the average effective dose being 110 μSv/month. The average effective dose for the cyclotron operator and nurse was 100 μSv/month. The dose for the full-time medical doctor and pharmacist was 25–40 μSv/month. At most nuclear medical facilities, γ emitters (e.g., 67Ga, 90Sr, 99mTc, 111In, 131I, and 204Tl) or positron emitters (e.g., 18F) are used. Thus, the lens dose would be comparable to the effective dose. The annual equivalent dose to the lens in radiological technologists was found to slightly exceed 1 mSv.
2.3.3. Internal radiation therapy
The JRIA working group for brachytherapy promotion published a guideline for safety management of permanent brachytherapy using 125I [45]. This guideline aims to comply with national regulations related to brachytherapy patient releases from medical facilities [46, 47], and follows ICRP Publication 98 [48]. For the brachytherapy, about 80 pieces of 125I seeds (13.1 MBq/seed) are used for a patient. In this case, the effective doses for medical doctors and nurses, estimated based on distance from a device, workload and activity of radiation sources, were 0.0033 mSv/procedure and 0.0015 mSv/procedure, respectively. The equivalent doses to the skin of medical doctors and nurses were 0.041 mSv/procedure and 0.007 mSv/procedure, respectively [45]. The effective dose and equivalent dose to the skin during preloading work were 0.0229 mSv/procedure and 0.206 mSv/procedure, respectively [45]. This guideline did not deal with the equivalent dose to the lens or radiation protection of the lens, but only recommended that medical staff should treat radiation sources with tweezers to protect the skin, reduce operating times, and shield radiations.
2.4. Nuclear workers
2.4.1. Commercial nuclear power plants (NPPs)
Japan has 42 operable nuclear power reactors (22 boiling water reactors, or BWRs, and 20 pressurized water reactors, or PWRs). These reactors are in 16 locations and are operated by 10 electric power companies [49, 50]. After the 1F-NPP accident in March 2011, the NRA in Japan issued new safety standards [49, 50]. The licensees had to take appropriate measures to restart the plants. Thus, no nuclear reactors were operated from 15 September 2013 to 11 August 2015. Currently, there are 13 units in the restart process, and 12 units are licensed from the NRA. Five reactors were in operation as of June 2017 (Sendai-1, Sendai-2, Ikata-3, Takahama 3 and Takahama 4 [49, 50]. In addition, 15 reactors, including 6 BWR reactors at 1F-NPP, are under decommissioning or are currently under review for decommissioning plans.
The occupational external exposure for workers at nuclear facilities is due to γ-rays emitted from activation products from corrosion such as 60Co, 58Co and 54Mn, and air-borne activation products such as 16N formed in the reactor core and preliminary cooling system during operation periods of NPPs, nuclear research and development facilities [51]. In BWRs, high-dose radiation fields are formed around steam turbine generators. However, the half-lives of these radionuclides are short. Thus, the external exposure of workers during regular inspections is mainly due to 0.6–1.3 MeV γ radiation emitted from activation products such as 60Co, 58Co and 54Mn that remain in the cooling systems and inside the plumbing of the system after the reactors are stopped [51]. Data for workers constantly exposed to radiation at nuclear facilities, such as radiation exposure doses, are registered in the Radiation Dose Registration Center of Radiation Effects Association.
In the NRA Act, the licensees are mandated to control the dose for radiation workers at nuclear facilities and to report the dose management to the NRA twice a year [52]. In FY 2014, beginning in April 2014, a total of 68 550 radiation workers were employed at commercial NPPs, among whom the number of employees at electric power companies and that of others such as contractors were 9891 and 58 659, respectively. The percentage of female workers was about 1% of total radiation workers [53]. The effective dose is assessed from Hp(10) measured at chest level. In dose management reports [52–54], only the effective dose distribution for the workers has been indicated. In NPPs, the workers are usually exposed to γ radiation. The exposure of the workers is approximately homogenous under the planned exposure situation, although exposure from the anterior part of the body is slightly predominant [55, 56]. Thus, this value would be considered comparable to the equivalent dose to the lens.
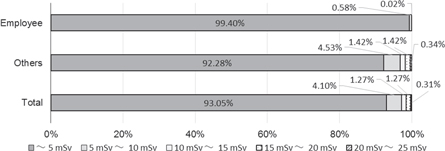
Figure 5 shows a trend in collective effective dose and the number of radiation workers employed at commercial NPPs for a decade, over FYs 2005–2014 [53]. Figure 6 shows the trend in average effective dose for the workers. Figure 7 shows the dose distributions of electric company employees and others in FY 2009. The dose received during emergency and recovery work during the 1F-NPP accident is included between FY 2010 and FY 2014. The collective dose ranged between 67 and 84 person-Sv in FY 2005–2009. The average effective doses for workers in BWRs and PWRs were 0.8–1.0 mSv and 1.1–1.3 mSv, respectively, in FY 2005–2009, during which time the maximum effective dose did not exceed 20 mSv/year. The effective dose for female workers was also under 5 mSv/3 months [53]. The average dose for the workers in BWRs in FY 2010–2014 was about 2 to 3 times as high as the dose before FY 2010. In FY 2012, the maximum effective dose was 54.1 mSv/year, although the emergency dose limit is 100 mSv (it was raised from 100 mSv to 250 mSv on 14 March 2011, and reduced from 250 mSv to 100 mSv on 1 November 2011). For female workers, the effective dose is also under 5 mSv/3 months except for those at 1F-NPP.
Figure 5. Trend of collective effective dose and number of radiation workers engaged at commercial nuclear power plants [53].
Download figure:
Standard image High-resolution imageFigure 6. Trend of average effective dose for radiation workers at commercial nuclear power plants by fiscal year [53].
Download figure:
Standard image High-resolution imageFigure 7. Distribution of effective dose at commercial nuclear power reactors in FY 2009 [53].
Download figure:
Standard image High-resolution imageIn the statistical dose data reported by the Radiation Dose Registration Center [53], the collective dose, average effective dose, and maximum dose for the workers were 87.0152 person-Sv (71 142 persons), 1.2 mSv/year, and 43.2 mSv/year in FY 2015, respectively.
The workers are exposed to β or inhomogeneous γ radiation during maintenance work. In these radiation fields, the workers usually wear protective face masks to prevent internal exposure, and active dosemeters, such as electronic dosemeters [57]. Thus, it must be noted that these values are not considered comparable to the equivalent dose to the lens in these radiation fields.
2.4.2. Other nuclear facilities
In FY 2014, the number of radiation workers employed at other nuclear facilities such as nuclear power reactor facilities at the research and development stage (2 facilities), nuclear reactor facilities for test and research (12 facilities), fuel fabrication facilities (6 facilities), fuel reprocessing facilities (2 facilities), radioactive waste burial and waste management facilities (4 facilities), and research reactors and nuclear fuel handling facilities belonging to universities, research institutes or companies (15 facilities), was about 18 500 persons [53]. The average effective dose for workers at these facilities was <0.1 mSv. The highest dose for workers was 4.7 mSv, at the fuel reprocessing facilities. In FY 2009, before the 1F-NPP accident, the average effective dose for workers at these facilities was <0.2 mSv and the maximum dose was 6.2 mSv at fuel reprocessing facilities. The effective dose for most workers did not exceed 5 mSv/year. Thus, the equivalent dose to the lens for workers during normal operation at these facilities would also be sufficiently lower than the new ICRP dose limit to the lens. There have been few tasks for which eye dosimetry was explicitly required, with a few exceptions at the Japan Atomic Energy Agency (JAEA). One such exception involved repair operations where only the upper bodies of workers were exposed in a partially opened hot cell. In that case, additional dosemeters were issued to clip on the workers' caps (i.e., at their foreheads); the average ratio of the forehead/torso γ dose was ∼2 [58]. Another such work situation is the glovebox operation in the fabrication process of mixed oxide fuel (containing 20%–30% plutonium by weight) for use in fast breeder reactors. The wearing of lead protective aprons is standard practice here, thus dose-rate gradients requiring workers to be monitored with a pair of dosemeters, so-called double dosimetry. The eye dose is computed from the γ Hp(10) of one dosemeter outside the apron (around the collar) plus the neutron Hp(10) of the other dosemeter under the apron. Eye exposure data from the past 10 years indicate that no workers have exceeded the annual dose of 20 mSv, with a maximum of 18.9 mSv recorded in 2008 [59].
2.5. Situation of radiation safety management at the TEPCO Fukushima Daiichi NPP
2.5.1. Current status of effective dose management
Works for recovery and reactor decommissioning were conducted after the 1F-NPP accident on 11 March 2011. Currently, around 10 000 workers per month conduct decommissioning tasks in the NPP [60]. Their exposed doses were high during the early phase of the accident, when the recovery operation was conducted. Internal exposure doses for some workers were especially high, mainly due to intake of 131I released from the reactors. The equivalent dose to the thyroid for two workers was distributed in the 10 000–15 000 mSv range, and these doses were the maximum [61].
The effective dose from external exposure was 199.42 mSv at maximum [62]. After the completion of Step 2* on 16 December 2011, the workers whose effective dose exceeded 100 mSv were not engaged in radiation work until 31 March 2016, following the official notice by the Ministry of Health, Labour and Welfare (MHLW), excluding some exceptional cases. Those exceptional workers were not engaged in radiation work from 1 May 2012 to 31 March 2016.
The effective dose limit under Japanese laws and ordinances for the emergency work, which was originally 100 mSv, was temporarily increased to 250 mSv, from 14 March to 16 December 2011 [63–65].
In Japan, the effective dose limit for emergency work was 100 mSv originally. The Japanese government enforced the laws and ordinances providing a higher limit of 250 mSv for an exceptional emergency, such as the 1F-NPP accident, on 1 April 2016 [66].
Footnote: * Step 2 was a process to attain stable cold shutdown conditions of the nuclear reactors as described on the 'Roadmap towards Restoration from the Accident at Fukushima Daiichi Nuclear Power Station' (Revised Edition of 17 October 2011 by the Government/Tokyo Electric Power Company Joint Office, Nuclear Emergency Response Headquarters).
2.5.2. Current status of equivalent dose assessment in the lens
Ideally, equivalent dose to the lens should be evaluated primarily using Hp(3), but it is evaluated using Hp(10) or Hp(0.07), whichever is the more appropriate value considering type and energy of radiation, according to an official notice of MHLW in Japan [67].
Radiation in the working environment at the 1F-NPP in the early phase of the accident was mainly γ-rays [68, 69]. Additionally, full-face masks were obligatory in all on-site areas in the 1F-NPP except for the inside of the Seismic Isolated Building. Hp(10) measured with alarm pocket dosemeters or other methods was therefore used for evaluating equivalent dose to the lens in early phase of the accident, until the establishment of 'β ray areas' (section 2.5.3).
Accordingly, maximum equivalent dose to the lens during the one-year period after the accident was the same as the effective dose from external exposure, which was 199.42 mSv [70].
2.5.3. Current status of exposure management in 90Sr/90Y dominant areas called 'β ray areas'
Water treatment plants to decontaminate and desalinate the contaminated water pooled in the basements of the turbine buildings were built, and started operation. Cesium absorption equipment was set in these water treatment plants, and 90Sr/90Y is the main source of radiation in the contaminated water after cesium removal.
In order to handle this situation, 90Sr/90Y dominant areas with an Hp(0.07)/Hp(10) ratio over 4 or suspected areas were defined as 'β ray areas' [71], and the management in the areas started in August 2011.
The effective dose limit was increased to 250 mSv, among the increased dose limits of occupational exposure in emergency exposure situations in 1F-NPP on 14 March 2011. Considering such changes in dose limits, the criteria of the β ray areas were set with the aim that the dose limit for the hands and feet (skin) would not be exceeded by work in the environment where 90Sr/90Y was the main radiation source.
Basic personal protection equipment (PPE) for work handling contaminated water in β ray areas is a full-face mask, nonwoven fabric coverall, an anorak, cotton gloves, double rubber gloves, socks, and long boots. In some cases, such as in demolition work of tanks with high β contamination, special rubber gloves or other special equipment are used. Wrist badges or ring badges should be used for exposure on extremities in addition to active personal dosemeters (alarm pocket dosemeters).
There was a case of detection of a high β dose. On 3 February 2012, oozing water was found at the joint of a concentrated water storage tank. This tank formed a part of a desalination system (RO). A high dose was confirmed locally in a gap between a concrete base and a flange of a tank, just below the coupling, with oozing. On the surface at this point, 22 mSv h−1 as Hp(10) and 2000 mSv h−1 as Hp(0.07) were detected [72].
In order to reduce dose inside the 1F-NPP and suppress rainwater penetrating underground, pavement coating of a wide area is underway. The coating of the land surface, other than the surrounding areas of Units 1 to 4, was conducted until March 2016 [73]. As a result of these dose reduction efforts, Hp(10) in the 1F-NPP was ≤5 μSv h−1 by the end of March 2016, except for the area surrounding the reactor buildings of Units 1 to 4 [74]. As the next step, since March 2016 the highly contaminated area surrounding the reactor buildings of Units 1 to 4 has been separated from other areas. The new operation rules using PPE according to areas with each contamination level were implemented after 8 March 2016, with the establishment of PPE exchange facilities [60, 75]. Under this new operation, PPE in the low contamination area was changed from coveralls with a nonwoven fabric to a normal work uniform or 1F-site-only clothes.
For lens dose assessment in 1F-NPP, Hp(0.07) on personal dosemeters attached to the chest (or abdomen, for women) is used without any conversion. In this case, the shielding by polycarbonate full-face masks is not taken into account, although all workers in high β ray areas use full-face masks. Thus, the dose assessment is considerably conservative compared with the true equivalent dose to the lens, and these conservative data have been recorded.
In the progress of the recovery operation, treatment of RO concentrated salt water with a high component of sea water, which is highly contaminated disposal water with a high concentration of strontium, was completed on 27 May 2015, except for residual water in the bottom of the tanks [76]. With this operation, the highly contaminated water with β sources of 90Sr/90Y mostly disappeared from the tanks. Additionally, absorption of cesium and strontium by dedicated equipment is continuing. The water processed by this equipment is called 'strontium-removed water', which is being re-purified by multi-nuclide removal equipment (ALPS) [77]. Thus, workplaces with 90Sr/90Y as the main radiation source have been reduced since 27 May 2015.
2.5.4. Problems from now on
The MHLW released its 'Report of the Expert Meeting on Epidemiological Studies Targeting Emergency Workers at the TEPCO Fukushima Daiichi Nuclear Power Plant' on 4 June 2014, which recommended including cataract and other non-cancerous diseases in future epidemiological studies whenever possible [78].
Considering the long-lasting reactor decommissioning work and the MHLW policy on epidemiological studies, establishment and operation of a more accurate method for dose assessment of the lens is expected. This dose assessment should appropriately include the shielding effects of full-face masks in addition to other relevant factors and should be an accurate assessment with minimal conservatism.
2.6. Discussion on occupational lens exposure
The lens dose distribution for Japanese radiation workers in various industrial and medical fields was discussed. The equivalent dose to the lens for most workers under normal operations is sufficiently lower than 20 mSv/year. However, the equivalent dose to the lens for some workers such as medical staff engaged in IVR and CT angiography procedures may exceed 20 mSv/year. It may be necessary to give high-dose workers more information on their own lens dose and appropriate dose reduction procedures using protectors. An additional important issue is radiation protection of workers when many workers are involved in unprecedented 1F-NPP decommissioning work over a long period of time. The radiation protection of the lens for the workers is no exception, and the optimized radiation protection of the lens should be considered, depending on the circumstances.
For workers who have already been provided with double dosimetry, it is unlikely that the lens dose limit reduction will have a significant impact on current practice or cause any confusion as to how lens dosimetry should be conducted. The double dosimetry practice has functioned well and taken root amongst workers over the past three decades, although it seems prudent to continue to disseminate the necessity of eye monitoring. Further discussion on the positioning of dosemeters for accurate measurement of lens exposure, as well as information regarding the dose correction method when wearing protective eyewear, should be tabled specifically for workers whose lens dose approaches or exceeds the reduced dose limit. Rather, a potentially greater impact is likely for other workers who are currently identified as receiving uniform exposure, and who are therefore not provided with double dosimetry. There may be more situations where lens dosimetry will be required; it is therefore necessary to investigate such situations by facility type and task type through a comprehensive field study, to estimate the potential increase in the number of workers who should be issued with an additional dosemeter for the eye. Other related issues involve the absence of individual dose compilation, except for the effective dose, at the national database of occupational exposure (available at the Radiation Dose Registration Center in Japan), and an extension of the database to the eye dose for easy data tracking.
3. Lens dose measurement in Japan
3.1. Historical changes in the lens dose limit
Radiation protection regulations in Japan underwent major changeovers in the policy and methodology of radiation dosimetry in 1989, whilst the lens dose limit remained almost unchanged. Until 1988, there was a lifetime whole-body dose limit of 5(N−18) rem, where N is the worker's age in years, and a dose limit of 3 rem per quarter for the head and trunk (excepting the skin and extremities). Technically, a maximum annual exposure of 12 rem (120 mSv) to the lens was therefore allowed. There had been no specific needs identified for eye monitoring, and the emphasis in radiological control was on avoiding unnecessary β exposure of the eye. In 1989, the concept of the effective dose equivalent was adopted, with a dose limit of 50 mSv/year, and a dose limit for the lens of 150 mSv/year, both of which were introduced in ICRP Publication 26 [79]. This implied a slight mitigation in the lens dose limit from 120 mSv (12 rem). The limit of 150 mSv was not changed in the 2001 revision to the regulations, which was based on ICRP Publication 60 [7].
3.2. Lens dose measurements
In 1989, to comply with the concept of the effective dose equivalent, the multiple dosimetry method (also known as multi-badging) was implemented, where multiple dosemeters are placed on designated parts of workers' bodies. Even under non-uniformly exposed conditions, a better estimate of the effective dose equivalent is obtained by summing the products of dosemeter readings and corresponding factors derived from weighting factors of the tissues and organs underlying each dosemeter location. This methodology is essentially the same as that proposed by Gill et al [80]. The position and number of dosemeters depend on the exposure situation, but in a typical situation, where a worker wears a protective apron covering from chest to thighs, the number of dosemeters is at least two: one on the trunk under the apron and the other at the collar above the apron. The latter dosemeter allows an estimate of dose equivalent to the lens (assuming no eye protection).
At the same time, operational quantities were introduced: the dose equivalents at depths of 10, 3 and 0.07 mm from the surface of the International Commission on Radiation Units and Measurements (ICRU) sphere phantom for photons and neutrons. The tabular data of these conversion coefficients from air kerma or particle fluence in frontal incidence exposure (i.e., anteroposterior geometry), cited from ICRP Publication 51 [81], were included in the appendix to the regulations. Thus, the numerical values in the normal incidence of today's 'ambient dose equivalent' or 'directional dose equivalent' were adopted as quantities to be measured regardless of whether they were used for area or personal monitoring. Angular dependence was interpreted as a characteristic inherent to the dosemeter, not to the dose equivalent itself; for example, a dosemeter with a less angular-dependent response over the frontal 2π space was regarded as ideal. For β particles, the measurement (7 mg cm−2 tissue absorbed dose) by an extrapolation chamber was the primary standard.
In the 2001 revision, multiple dosimetry was continued, but the system of operational quantities was redesigned on the basis of ICRP Publication 74 [82], and personal dosemeters were re-calibrated to indicate the personal dose equivalent, Hp(10) and Hp(0.07), defined in the ICRU slab phantom. Moreover, the requirement for Hp(3) measurement was exempted, and thus proper values of either Hp(10) or Hp(0.07) measured with personal dosemeters were recorded as a surrogate of Hp(3).
This exemption is not really problematic for strongly penetrating radiations such as photons and neutrons, since they produce no marked differences in depth-dose profile. For weakly penetrating β particles, however, unconditional assignment of the Hp(0.07) to the lens dose results in overestimation. Criteria for when and how to use multiple dosimetry depend on the degree of non-uniform exposure over the body trunk and the magnitude of the dose delivered. At present, in the case of wearing a protective apron, one frequently-used guide is whether an expected dose ratio between the inside and outside of the apron exceeds 3, which is the ratio of 150 mSv (derived from equivalent dose limit for the lens of 150 mSv/year) to 50 mSv (derived from effective dose limit of 20 mSv/year averaged over 5 y with no single year exceeding 50 mSv/year).
Sets of two dosemeters for double dosimetry are available from several dosimetry services, among which RPLDs or OSLDs are routinely used in the medical sector. Although the current Japanese Industrial Standard (JIS) on personal dosemeters does not state any requirement for Hp(3), its revision is underway to incorporate Hp(3) dosimetry in accordance with the latest international standard, International Electrotechnical Commission (IEC) 62387:2012 [83].
3.3. Discussion on lens dose measurement
It is expected that the Hp(3) measurement requirement will be incorporated into the regulation again in the near future. It appears that application of the new set of conversion coefficients obtained in the head-geometry computation model and the associated calibration phantom (e.g., a 20 cm diameter cylinder) would not cause major changes in currently recorded eye doses for γ rays and neutrons. However, this is not the case for β particles. To be more precise, this is an urgent problem within the current regulations, not a future challenge.
As mentioned in section 2.5, the current policy on lens dosimetry – Hp(0.07) instead of Hp(3) is assigned as the eye dose of record in a β-dominant field – came under criticism that absolute adherence to the prescribed regulations for routine monitoring had to be recognized as unrealistic for special monitoring during recovery operations in the aftermath of the 1F-NPP accident. The β Hp(3) dosimetry for 1F-NPP workers should be based on a conventional method to exclude extra conservatism.
Before 2000, β Hp(3) had been converted (if necessary) from β Hp(0.07), using a correction factor customarily determined from experiments in which the β elements with a 300 mg cm−2 density thickness filter of a personal dosemeter were exposed to β radiations. Moreover, at present, a survey meter specifically designed to measure H'(0.07) is on the market, using an air ionization chamber constructed of a wide, thin window with a shallow sensitive volume, together with an optional detachable 3 mm thick window cover. With such survey instruments, comparative measurements of β Hp(0.07) and β Hp(3) dose rates in typical workplaces at the 1F-NPP site can provide reliable and practical Hp(3)/Hp(0.07) ratios. This will allow better estimates of β Hp(3) to be converted from β Hp(0.07), and can assist the retrospective interpretation of dosimetry results. Development and establishment of the 300 mg cm−2 tissue absorbed dose (rate) standard for β radiation is continuing at the National Metrology Institute of Japan [84].
4. Epidemiological and biological studies of ionizing radiation cataracts in Japan
The Radiation Effects Research Foundation (RERF) in Hiroshima and Nagasaki is conducting epidemiological studies in atomic bomb (A-bomb) survivors. Over the past four decades, A-bomb data have been the gold standard in estimating a dose threshold for acute exposure for radiation protection purposes. For instance, ICRP recommended 0.5 Gy as an acute threshold for cataracts in 2011, in which two papers played major roles (one is on cataract prevalence [85], the other on postoperative cataract prevalence [86]). RERF has published two more papers since then, one on cataract surgery incidence [87] and the other on cataract surgery prevalence [88]. Preliminary analysis has documented a significant increase in cataract surgery prevalence in all minor homozygotes of three ataxia telangiectasia mutated (ATM) haplotypes [89], implying genetic susceptibility to radiation cataracts [90, 91].
Kanazawa Medical University in Kanazawa is performing epidemiological studies in workers following the 1F-NPP accident. Among 507 workers whose effective dose (cumulative dose received from the start of employment by TEPCO until July 2014) exceeded 50 mSv, the prevalence of lenticular changes at 4 years after the accident was 2.6% (95% confidence intervals, 1.2%–3.9%) for cortical cataracts, 0.6% (0.1%–1.7%) for cortical cataracts inside the central 3 mm circular pupillary area, 0.4% (0.04%–1.4%) for retrodots, 1.8% (0.6%–2.9%) for water clefts, 13.0% (10.1%–15.9%) for posterior subcapsular (PSC) vacuoles outside the central 3 mm circular pupillary area, 5.9% (3.9%–8.0%) for PSC vacuoles inside the central 3 mm circular pupillary area, and 0.0% for nuclear cataracts and PSC cataracts. The mean effective dose was 87.23 mSv, and 135 workers out of 507 exceeded 100 mSv. The association between the aforementioned lenticular changes and effective dose was statistically insignificant. Investigators of this work raised the possibility that PSC vacuoles inside the central 3 mm circular pupillary area may be increasing and may progress to PSC cataracts, highlighting a need for long-term follow-up [92].
The Central Research Institute of the Electric Power Industry (CRIEPI) in Tokyo is carrying out a biological study using primary human lens epithelial cells HLEC1 in vitro. Ionizing irradiation was found not only to inactivate the clonogenic potential of HLEC1 cells but also to stimulate proliferation in its subpopulation [93], and such enhanced proliferation of lens epithelial cells was also recently reported to occur in vivo [94].
5. Conclusions and perspectives
5.1. From epidemiological viewpoints
As mentioned above, epidemiological studies on radiation cataracts in A-bomb survivors and the Fukushima workers are continuing. To the best of our knowledge, however, no other studies have started in Japan, such as in medical sectors, necessitating the gathering of more data from among the Japanese population. Moreover, the epidemiological provability of radiation effects depends on levels of the variations in background risk [95]. In this regard, nationwide Japanese background data are unavailable, but one paper reported a difference in the prevalence and types of cataracts in two areas of Japan (892 residents of Noto and 314 residents of Amami) [96]. For example, the prevalence of all cataracts (cortical, nuclear, PSC, and mixed types) and that of cortical cataracts was 36.5% and 34.0% in people aged in their 50s (i.e., 93.2% of cataracts observed were cortical), 65.6% and 51.0% in people in their 60s, 84.3% and 39.8% in people in their 70s, and 100% and 23.3% in people in their 80s and above in Noto; 54.0% and 46.1% in the 50s, 83.1% and 46.9% in the 60s, 96.9% and 18.5% in the 70s, and 100% and 5.6% in the 80s and above in Amami, respectively. There is also a report suggesting that ultraviolet light B (UVB) exposure may account for a difference in A-bomb cataracts between Hiroshima and Nagasaki [97]. These underline a need for a nationwide survey to obtain the Japanese background data.
5.2. From radiation protection and dosimetry viewpoints
In the industrial field, the effective dose and equivalent dose to the lens in non-destructive inspection workers are higher than those for other industrial workers. In the general medical field, the equivalent dose to the lens for some workers may exceed 20 mSv/year (100 mSv/5 years). However, the proportion of workers whose equivalent dose exceeds 20 mSv is low. Care should be taken by doctors and radiological technologists during IVR procedures, CT angiography procedures, and nuclear medicine examinations to protect the lens by using ceiling shields and protective eye wear.
There are many results for the monitoring and dose measurements of medical staff. However, large and continuous projects on the occupational exposure of the lens are limited.
At nuclear facilities, radiation workers are generally exposed to homogeneous radiation fields under normal operating conditions. The effective dose for most workers employed at nuclear facilities, except for emergency workers at 1F-NPP, would be sufficiently lower than 20 mSv/year. Thus, the equivalent dose to the lens of most nuclear workers would be low, like the effective dose. The licensees must manage the low dose to radiation workers adequately and reasonably. At 1F-NPP, as mentioned before, the β lens doses in some workers are significantly overestimated, due to the current policy of the lens dosimetry, Hp(0.07) instead of Hp(3), as well as no account being taken of the protection afforded by the mask. Realistic estimates of the lens dose should be made, so as not to overestimate the worker's risk. The dose distribution in workplaces and the personal dose for workers is investigated for each type of work, and equivalent dose to the lens should be estimated by using the latest data. It is required by the legislation and regulations to record the total accumulated occupational effective dose for radiation workers as well as to perform regular medical checks, such as blood tests, and skin and eye examinations. However, workers can omit an eye examination when the doctors consider it is unnecessary, and measurement of the equivalent dose to the lens has not been made until now. In addition, the dose for workers (except for nuclear workers) is not unitarily managed. Also, the medical exposure would not be negligible when estimating the accumulated individual equivalent dose to the lens, when workers may have many opportunities for medical x-ray examinations as patients.
In Japan, the ICRP 2007 Recommendation and new ICRP dose limit for the lens have not yet been incorporated into national regulations. In January 2016, the Integrated Regulatory Review Service (IRRS) mission for the government and/or NRA was conducted by IAEA [98]. The IRRS team made recommendations and suggestions, indicating that improvements are necessary or desirable in order to progressively align the framework with the IAEA Basic Safety Standard [99]. The NRA listed improvements of 27 issues, including discussion of the equivalent dose limit to the lens as the intermediate-term objective from FY 2015 to FY 2019, and is planning to start relevant safety research of radiation protection [100].
Many studies on radiation effects, dosimetry and radiation protection of the lens will need to be conducted systematically and widely; they would contribute to future discussion on the dose limit to the lens in national regulations. For example, it would be necessary to collect more information on actual exposure to the lens of not only workers in 1F-NNP but also nuclear workers carrying out more recent maintenance work during normal operations in nuclear power plants. Furthermore, we would also need to discuss how to measure and evaluate the equivalent dose to the lens when protective equipment or eyewear is used, and to develop the most suitable radiation protectors in the medical field.