Abstract
MnAl alloys are very promising rare-earth-free permanent magnets. Nanocrystalline microstructures can have beneficial effects on the properties of magnetic MnAl alloys. In the present work we examined multiple routes to process MnAl alloys and studied the effects of milling on Mn-46 at.% Al powders. Mn54Al46 was produced via gas atomization, melt spinning, and rapid solidification rate processing. It was then mechanically milled using a water-cooled Union Process attritor for times up to 20 h. X-ray diffraction patterns showed the presence of mostly the high-temperature ε-phase with significant amounts of the equilibrium γ2 and β phases in both the cast and milled particulates. The powders were annealed for various temperatures and times in order to obtain the ferromagnetic τ-phase. Magnetic measurements of the optimally annealed powders showed a coercivity of 3.62 kOe and saturation magnetization of 59.8 emu g−1 for mechanically milled gas-atomized powder annealed at 500 °C for 30 min.
Export citation and abstract BibTeX RIS
1. Introduction
High performance permanent magnets (PM) have become essential components in everyday life. They are used in automotive, magnetic recording, navigation, wind generator turbine applications and countless other technologies. Rare earth (RE)—transition metal intermetallics are the most powerful PM materials at present [1–3]. Their exceptional magnetic properties arise from the favorable combination of RE metals (Nd, Pr, Sm) with transition metals (Fe, Co), particularly in Nd–Fe–Co and Sm–Co based magnets. The energy product ranges from 15 to 32 MGOe for Sm–Co based magnets while the Nd2Fe14B have the highest energy product approaching 56 MGOe [4]. While these RE magnets have the highest energy product (BH)max of any material they are very expensive (as of May 2013: Dy ∼ $600 kg−1, Nd ∼ $90 kg−1 and Sm ∼ $40 kg−1) and can have various problems. For example, sintered Nd2Fe14B is vulnerable to grain boundary corrosion requiring nickel or copper/nickel plating or lacquer coating [5]. Polymer-bonded Nd2Fe14B magnets do not suffer from this problem but have a significantly lower energy product due to the polymer matrix. Sm–Co magnets also do not suffer from corrosion and can be used at higher temperatures (up to ∼350 °C), but are quite brittle, prone to chipping and can fracture from thermal shock [6]. Further issues with these materials are that over 95% of REs are produced in China, which is curtailing exports [7].
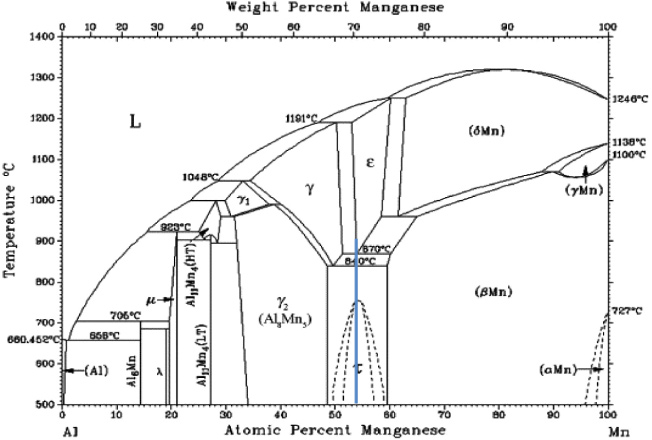
Less expensive magnets are more commonly used, but these magnets generally have lower coercivity, HC, i.e., their internal magnetization is susceptible to alteration by nearby magnetic fields. For example, Alnico alloys, which contain large amounts of nickel, cobalt and iron and small amounts of aluminum, copper and titanium, have HC in the range of 0.6–2 kOe [8], which makes exposure to significant demagnetizing fields undesirable. Similarly, ferrites, which are predominantly iron oxides, are the cheapest and most popular magnets [8], but they have both low HC (1.6–3.4 kOe) and low values of saturation magnetization, Ms, e.g. BaO ⋅6Fe2O3 has an Ms of ∼380 emu cm−3. The ferromagnetic τ-phase in the MnAl system, first reported by Kono [9] and Koch et al [10], continues to attract attention since it has an attractive combination of magnetic properties for modern technological applications. Even though neither manganese nor aluminum are ferromagnetic, the metastable tetragonal τ-phase, see figure 1, with a composition around Mn-46 at.% Al, the composition at which the eutectoid transformation occurs at 870 °C, can be produced with a high remanence, coercivity, and saturation magnetization [11]. In contrast, at equilibrium this alloy consists of the non-magnetic β phase and γ2 phases. The ε-phase in the Al–Mn system, from which the ordered ferromagnetic τ-phase is derived, is antiferromagnetic with a Néel temperature of 90 K [12, 13]. MnAl has a theoretical energy product, (BH)max, of 12 MGOe, a density of 5200 kg m−3 (by comparison Sm–Co magnets have a density of ∼8300 kg m−3), and, thus, a high density-compensated (BH)max of 2.31 KGOe m3 kg−1, see table 1. Its magnetic properties are potentially superior to those of conventional hard ferrites, Alnicos, and Fe–Cr–Co alloys. In addition, τ-MnAl does not suffer from the issues associated with RE magnets outlined above. The low costs and excellent availabilities of the Mn and Al, high specific strength [14], strong uniaxial magnetocrystalline anisotropy (106 J m−3) [15], as well as the excellent corrosion resistance [16], all make τ-MnAl particularly attractive.
Figure 1. Al–Mn binary phase diagram adapted from [10] showing the extent of the metastable τ phase region. The vertical line shows the Mn-46 at.% Al target composition. The eutectoid transformation at 870 °C occurs at this composition.
Download figure:
Standard image High-resolution imageTable 1. Comparison of the estimated maximum energy product, density and density-compensated maximum energy product for several classes of permanent magnets.
| (BH)max(MGOe) | Density (kg m−3) | (BH)max/density(kGOe m3 kg−1) | References | |
|---|---|---|---|---|
| Nd2Fe14B | 45 | 7600 | 5.92 | [28] |
| Sm–Co | 30 | 8300 | 3.61 | [29] |
| τ-MnAl | 12 | 5200 | 2.31 | [18] |
| AlNiCo | 6 | 7000 | 0.86 | [30] |
| Ferrites | 4.5 | 5000 | 0.90 | [31] |
Recently, nanocrystalline τ-MnAl powders with excellent magnetic properties (Ms = 89 emu g−1 and Hc = 4.8 kOe) were produced by mechanically milling powders of the high temperature ε-phase, formed by arc melting followed by pulverization, until they were nanocrystalline, followed by annealing [17–19]. This was quite different to the approach undertaken by others, i.e. producing the τ-phase and then milling it to produce nanocrystalline material[11, 20–22]. The origin of the high Hc may be the nanostructure and/or the presence of small amounts of the equilibrium γ2 and β phases (figure 1), which could pin the magnetic domain walls [19].
Producing τ-MnAl starting with pulverized arc-melted ingots, as described above, is not an easily scalable process. The Al–Mn phase diagram [23] shown in figure 1 shows several Al–Mn intermetallic compounds with the high temperature ε-phase existing in the range 53–60 at.% Mn. The difficulty with producing the ε-phase, from which the L10 τ-phase forms, is that it is only in equilibrium at >870 °C. Thus, it has to be formed via non-equilibrium processing techniques. In the present paper, we examine the production of τ-MnAl particulates using three different techniques, i.e. gas atomization, the Pratt and Whitney rapid solidification rate (RSR) process and melting spinning. We then examined the effects on the magnetic properties of milling and annealing Mn-46 at.% Al powders.
2. Experimental details
Mn-46 at.% Al particulates were produced by three different methods. First, batches of Mn-46 at.% Al powder were gas-atomized under argon by Allegheny Technologies Incorporated (ATI Powder Metals, 6515 Steubenville Pike, Pittsburgh, PA 15205). Each batch was separately chemically analyzed by ATI and shown to be within ±0.4 at.% of the required composition.
Second, Mn-46 at.% Al Powder was cast into the ribbon using a conventional melt spin caster under argon. Two batches of melt-spun ribbons were fabricated at the Materials Science and Engineering Department, Carnegie Mellon University. One batch was cast at normal wheel speed that produced short ribbons. The second batch was cast at a slightly slower wheel speed, which resulted in more continuous ribbon.
Third, powders were produced using the RSR process, developed originally by Pratt and Whitney, West Palm Beach, FL, but performed by Ervin Technologies, Inc. Tecumseh, MI. In this process, molten alloy is poured onto the face of a water-cooled copper dish rotating at ∼30 000 rpm under an argon atmosphere. The process breaks up the molten stream into droplets that spray outwards from the rotating disk where the molten droplets are rapidly cooled by high-speed argon jets. The key difference between the melt-spun material and that produced by the RSR process is that in the former molten metal impinges on the circumferential surface of the spinning disk while in the latter the motel metal impinges on the face of the disk. This results in very thin, long rapidly solidified ribbons produced though melt spinning and rapidly solidified powders produced by the RSR process.
The RSR powder was analyzed, by Ervin Technologies, Inc. Following acid dissolution, inductively coupled plasma atomic emission spectroscopy was employed to determine the composition of the sample, which was found to be Mn-47 at.% Al.
Nanocrystalline ε-MnAl powders were produced through mechanically milling (MM) the gas-atomized powders using a water-cooled Union Process 1SD Svegari attritor. The milling process was also performed under Ar to prevent any oxidation. Typically a rotation speed of ∼700 rpm was used with hardened steel balls for 2–20 h with a 10:1 ball-to-powder ratio.
The sizes and morphologies of both the as-received particulates and the milled powders were determined using secondary electron imaging in a FEI field emission gun (FEG) XL30TM scanning electron microscope (SEM). The phases present in each of the particulates were determined using a Rigaku DMax rotating anode x-ray diffraction (XRD) system with a Cu target. Conditions for XRD were the following; tube: Cu Kα = 0.154 06 nm, filter: Ni, accelerating voltage: 40 kV, current: 20 mA and two theta scan range: 5°–90°. The magnetic properties of the powders were measured with a Lakeshore Instruments 7300 VSM that can apply fields up to 10 kOe.
3. Results and discussion
3.1. As-cast materials
Figures 2(a) and (b) show secondary electron images of the as-received gas-atomized powders and the powders after milling for 20 h. The as-received powders were spherical with sizes ranging from <20 to 45 μm. The particle size decreased gradually with increasing milling time and some were welded during the milling, see figure 2(b). The shape of these particles is quite irregular.
Figure 2. Secondary electron images of (a) as-received gas-atomized powders and (b) powders after milling for 20 h.
Download figure:
Standard image High-resolution imageFigure 3 shows x-ray diffraction patterns of both the as-received MnAl powder and the powder mechanically milled for 10, 12 and 20 h. Comparison of the x-ray diffraction pattern from the as-received powder with calculated diffraction patterns for ε, ε', γ2, β and τ phases shows that the powders consisted mostly of the ε phase with significant amounts of the equilibrium γ2 and β phases. There was no evidence of the τ phase. From the XRD data, it is found that the as-received powder was ∼66% ε-phase with the rest being the γ2 and β phases.
Figure 3. X-ray diffraction patterns of as-received gas-atomized powder (bottom) and after milling the powders for 10, 12 or 20 h (top).
Download figure:
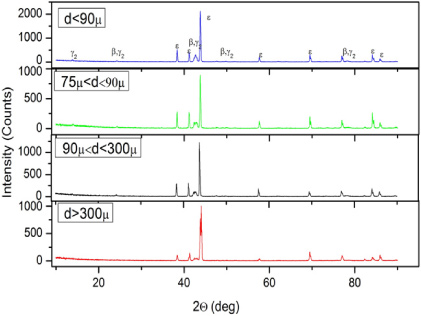
Standard image High-resolution imageIn order to aid analysis of the as-received powders to determine why no τ phase was present but significant amounts of the γ2, and β phases were present, the powders were sieved into different size ranges. The Ro-Tap® Electronic Test Sieve Shaker was used for this process. It might be thought that finer powders would cool more rapidly and, thus, contain less of the equilibrium phases. However, the x-ray diffraction data shows exactly the opposite, i.e. finer powders have more of the equilibrium γ2, and β phases, see figure 4. This behavior likely arises because during gas atomization in argon, which has relatively poor thermal conductivity, fine powders can, by Brownian motion, not end up in the gas stream and, thus, not receive significant cooling. The results indicate that gas atomization in argon may not be the best processing route to produce 100% ε-phase MnAl powders.
Figure 4. X-ray diffraction patterns from the gas-atomized powders sieved into three different size ranges, i.e. <20, 20–38 and 38−45 μm.
Download figure:
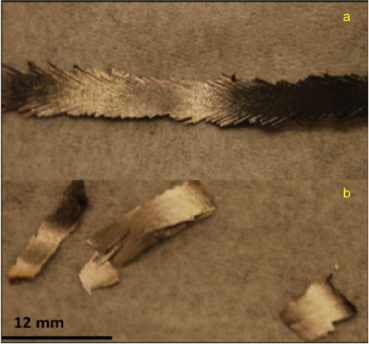
Standard image High-resolution imageFigure 5 shows optical micrographs of the two sets of ribbons produced by melt spinning while figure 6 shows x-ray diffraction patterns from the ribbons with the XRD pattern of the as-received gas-atomized powder for comparison. It can be seen that for both sets of ribbons the high temperature ε phase is mostly present with minimal amounts of the γ2 and β phases. This is consistent with the observations of others, who have found essentially the ε phase after melt spinning [24, 25]. The ribbons processed with the slower speed turns out to be continuous but with more pronounced τ-phase peaks.
Figure 5. Optical micrographs of melt-spun Mn-46 at.% Al: (a) long ribbons and (b) short ribbons.
Download figure:
Standard image High-resolution imageFigure 6. X-ray diffraction patterns of melt-spun Mn-46 at.% Al: (a) long ribbons and (b) short ribbons. The x-ray diffraction pattern for gas-atomized powders is shown for comparison.
Download figure:
Standard image High-resolution imageFigure 7(a), shows scanning electron micrographs of the powders produced using the RSR process, which consist of spherical particles with some large flakes of MnAl. The flakes arose because the processing conditions were not optimized. With optimization, the flakes would no longer be present and the particle size distribution would be narrower.
Figure 7. Secondary electron images of: (a) powder produced by the RSR process and (b) powder milled in attritor for 20 h.
Download figure:
Standard image High-resolution imageFigure 8 shows x-ray diffraction patterns obtained from the RSR powder sieved into different size ranges by Ervin Technologies, Inc. Note that while β and γ2 phases are again present in the RSR powders, the amounts are much less (<10%) than in the gas-atomized powders previously produced. Interestingly, as with the gas-atomized powders, the amounts of the β and γ2 phases decrease with increasing particle size. It is believed that the high cooling rate essentially by-passed the formation of a high temperature phase, allowing lower temperature phases to form directly in smaller particles. This issue can easily be fixed by having the water-cooled copper dish rotate more slowly, thus producing larger particle sizes and slower cooling rates. It also suggests that gas atomization may be a viable approach if larger sized particles are produced.
Figure 8. X-ray diffraction patterns of Mn-47 at.% Al powders produced by the RSR process separated into different size ranges.
Download figure:
Standard image High-resolution image3.2. Mechanically milled materials
The MM gas-atomized powders showed the same phases as the as-received gas-atomized powders (figure 3), but the diffraction peaks become increasingly broadened with increasing milling time, indicating the formation of very fine grain structures. The average grain size (and lattice strain) was calculated from the corrected full width at half maximum of each diffracted peak, βsample, for the powders using the Hall–Williamson method [26]. The grain size for the 20 h milled MnAl, calculated from (002) peak was 12 nm.
Annealing of the milled powder transforms the ε-phase into the metastable τ phase; the equilibrium γ2, and β phases are still present in essentially the same amounts as before annealing, see figure 9. In other words, none of the ε-phase was transformed to the equilibrium γ2, and β phases.
Figure 9. Comparison of the x-ray diffraction patterns of gas-atomized powder in the as-received condition (bottom) and after milling for either 8 or 20 h (top) and subsequent annealing at 400 °C.
Download figure:
Standard image High-resolution imageThe RSR powder was also mechanically milled under the same conditions and the milling transformed the powder in to a fine grained structure (see figure 10). A grain size of 45 nm was determined for the ε phase using x-ray line broadening analysis of powder milled for 20 h. Annealing of the MM powder shows transformation of ε phase into the metastable τ phase and the equilibrium γ2, and β phases. The reason that γ2, and β phases were formed on annealing the RSR powder but not the gas-atomized powder may have been because of differences in composition of the powders, i.e., the RSR powders contained 47 at.% Al while the gas-atomized powders contained 46 at.% Al.
Figure 10. X-ray diffraction patterns of RSR powder (<90μ) after 20 h MM conditions (bottom) and after annealing at 450 °C.
Download figure:
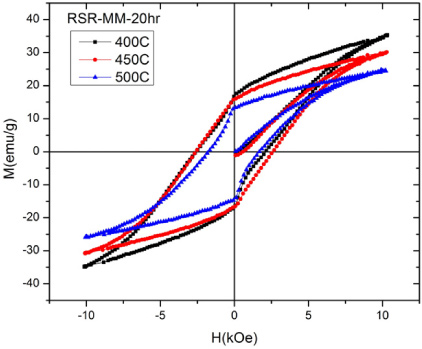
Standard image High-resolution imageFigure 11 shows the magnetization as a function of applied magnetic field for gas-atomized powders mechanically milled for 20 h in the attritor and annealed for 30 min at various temperatures. The best Ms value (59.8 emu g−1) and best Hc value (3.62 kOe) were for a sample annealed at 500 °C for 30 min. Both of these values are less than the values of Ms = 78.3 emu g−1 and Hc = 4.32 kOe obtained by Zeng et al [19]. Figure 12 shows the M−H curves for the RSR powder mechanically milled for 20 h under the same conditions as that for the gas-atomized powder. The best MS value (35.2 emu g−1) and HC value (2.7 kOe) was less than the values obtained for gas-atomized powder. The decrease in MS of MnAl is related to the fact that the magnetic τ-phase decreases with annealing temperatures. In the ideal stoichiometric CuAu lattice the A and B atoms occupy alternating planes perpendicular to the C-axis, Mn atoms on A sites couple ferromagnetically to each other, but antiferromagnetically to Mn atoms on B sites. Disorder, with Mn atoms occupying Al sites, results in a reduced magnetization. However, it is worth noting that the values for both the gas-atomized and RSR powders were measured using a maximum applied field of 10 kOe on a VSM and that these powders were determined to be Mn53Al47. Measurements by Zeng et al [19] using a maximum applied field of 15 kOe on a VSM yielded Ms = 89 emu g−1 and Hc = 4.8 kOe. The measurements on the VSM under the lower field strength do not saturate the specimens and so an accurate Ms value cannot be determined. However, the Ms measurements of the gas-atomized powder using a VSM are better than those obtained by Zeng et al [19] when measured using a VSM. This is somewhat surprising since the gas-atomized powder does not contain 100% ε-phase as in the case of arc-melted MnAl [18, 19]. The reason for the lower measured value of Hc is unclear. It is hypothesized that the high HC was obtained in milled and annealed material produced from arc-melted particulates due to the nanoscale distribution of the γ2 and β phases [27]. The values obtained here lend some credence to that hypothesis since the γ2 and β phases present in the annealed powders are probably quite coarse since they formed during cooling following the gas atomization.
Figure 11. Magnetization versus applied magnetic field for gas-atomized powders mechanically milled for 20 h and annealed for 30 min at various temperatures.
Download figure:
Standard image High-resolution imageFigure 12. Magnetization versus applied magnetic field for RSR powders mechanically milled for 20 h and annealed for 30 min at various temperatures.
Download figure:
Standard image High-resolution image4. Summary
τ-MnAl powders were produced by three non-equilibrium processes: gas atomization, melt spinning, and rapid solidification processing, followed by mechanical milling and subsequent heating. Although the as-received gas-atomized powders contained unwanted γ2 and β phases, they still consist largely of the ε phase. We achieved a Hc value of 3.62 kOe and a Ms value 59.8 emu g−1 for a sample annealed at 500 °C for 30 min. It should also be noted here that the τ-MnAl is isotropic in the present study. Although the gas-atomized powder as a whole contained only 66% ε-phase, higher volume fractions of the equilibrium phases were found in finer powders. Thus, the issue with powders appears to be that the cooling rate is so high that formation of the high temperature phase often does not occur and that the low temperature equilibrium γ2 and β phases form directly from the melt. The metastable τ-phase would not form this way since it transforms from the ε phase and not directly from the melt. This means larger particles at slower cooling rates, which are more easily and cheaply produced, can be used.
Acknowledgments
This research was funded by the US Department of Energy, Advanced Research Projects Agency-Energy (ARPA-E) through REACT program contract no. DE-AR0000188 with Dartmouth College.