Abstract
Atomic friction on hydrogenated graphene is investigated using molecular dynamics simulations. Hydrogenation is found to increase friction significantly, and the atomic-level information provided by the simulations reveals that atomic roughness induced by hydrogenation is the primary cause of the friction enhancement. Other proposed mechanisms, specifically adhesion and rigidity, are excluded based on the simulation results and analyses performed using the Prandtl–Tomlinson model. In addition, it is found that friction does not monotonically increase with hydrogen coverage on the graphene surface; instead, a maximum friction is observed at a hydrogen coverage between 5 and 10%.
Export citation and abstract BibTeX RIS
1. Introduction
For decades, nanotribology has been attracting significant attention due to its scientific importance and practical potential [1, 2]. Graphene and graphite are ideal materials for friction measurements at the nanoscale because these materials are chemically inert and can be easily cleaved to form an atomically flat surface. Indeed, the first measurement of atomic stick–slip friction by atomic force microscopy (AFM) was conducted on graphite [3]. Many interesting tribological phenomena have been observed on graphene and graphite since then.
Graphene and graphite have also been the focus of studies trying to find ways to control atomic friction. For example, superlubricity [4], a state of surprisingly low friction, was achieved on graphite both at the nano- and microscales [5, 6]. Also, it was shown that friction monotonically increased as the number of graphene layers decreased. This friction enhancement was attributed to a puckering effect [7]. In another case, a negative friction coefficient was observed on oxidized graphite surfaces [8], which is in sharp contrast with the well-known Amonton's law, where friction linearly increases with normal load. Finally, a recent study revealed that graphene chemically modified with fluorine and hydrogen can give rise to a much larger friction [9, 10]. In that work, a phenomenological interpretation was given: when terminating graphene surfaces with hydrogen or fluorine, the surface becomes more rigid, causing an increase in friction. This phenomenological explanation seems promising, but there is still a lack of atomic-level evidence. In general, there is significant potential for controlling friction at the atomic scale, not just on graphite and graphene, but also on other solid lubricants, such as MoS2, that are already widely used [11].
In this study, we apply molecular dynamics (MD) simulation to explore the atomic-level origin of the enhanced friction on hydrogenated graphene. Atomistic simulation is a powerful supplementary tool for experiments and provides valuable atomic-level information about the physical processes occurring in the buried interface [12]. MD simulations have been successfully applied to investigate friction on pristine graphene [13–17]. Here we present results of MD simulations of atomic friction on hydrogenated graphene, and reveal that enhanced friction is correlated with the atomic roughness induced by hydrogenation.
2. Methodology
The atomistic model of an AFM measurement is illustrated in figure 1. In the simulations, hemispherical diamond tips with radii of 2–4 nm slide against the hydrogenated graphene. There are four graphene layers in the substrate, the bottommost of which is fixed. The topmost three layers of the diamond tip are treated as rigid and dragged by a virtual atom with a constant velocity through a harmonic spring. The harmonic spring has stiffness k = 10 N m−1 and is used to mimic the compliance of an AFM system. A detailed discussion of the role of compliance in atomic friction and how to accurately capture the effective stiffness of an AFM measurement in MD simulation can be found in [12]. The spring is in effect along directions parallel to the surface. No spring is applied in the normal direction. Instead, a constant force is added on the top of the diamond tip to simulate the normal load in AFM measurements. To control the temperature of the system, the Langevin thermostat is applied to four atomic layers in the middle of the tip and one atomic layer in the middle of the substrate. Simulations are run at both 10 and 300 K, and similar trends are observed in both cases. To filter out the noise stemming from thermal effects, the results shown in this paper correspond to simulations at 10 K. The hydrogen atoms are randomly distributed on the top of the graphene surface. Hydrogen coverage is defined as the ratio of the number of hydrogenated carbons to the total number of carbons in the graphene, and coverages ranging from 1% to 40% are investigated. The AIREBO [18] potential, which is known to be able to describe the hydrocarbon bonds as well as the van der Waals forces, is employed to simulate the interaction of atoms within the tip and substrate. The interaction between the tip and substrate is simulated by the Leonard-Jones (LJ) potential with parameters adopted from [18] as well. Simulations are carried out using the molecular dynamics simulation package LAMMPS [19].
Figure 1. Molecular dynamics simulation of a diamond tip sliding against hydrogenated graphene. The inset shows hydrogenated graphene and its 3D structure due to the sp3 carbon bond.
Download figure:
Standard image High-resolution image3. Results
Figure 2 shows the MD-predicted friction over a range of loads for hydrogenated and pristine graphene. The friction on pristine graphene is relatively low and increases only slightly with normal load, which is consistent with previous experimental and simulation-based investigations [5, 20, 9, 17, 21]. In contrast, the hydrogenated surface gives rise to a dramatically enhanced friction. Indeed, friction enhancement was recently observed on fluorinated graphene [9], as well as hydrogenated and oxidized graphene [10, 22], through AFM measurements. The inset of figure 2 reveals another effect of hydrogenation. On pristine graphene, the lattice spacing of the hexagonal structure of graphene can be captured through the periodicity of the friction trace. However, on hydrogenated graphene, although we still observe atomic stick–slip friction, the periodicity of these patterns varies along the sliding distance due to the random location of hydrogen atoms. Thus one cannot acquire the hexagonal lattice structure, which is also consistent with the experimental observations in [10].
Figure 2. Load dependence of friction on hydrogenated graphene with 10% hydrogen coverage (circles) and on pristine graphene (squares) for a 3 nm radius tip. The lines are linear fits to the data. Friction traces measured at a normal load of 20 nN are shown in the inset.
Download figure:
Standard image High-resolution imageSince the simulation and experiment are consistent, we further seek the underlying mechanism for the frictional enhancement. It is well known that an increase of adhesive force, the interaction in the normal direction between the tip and substrate, can lead to larger friction [23, 8], and adhesion has been used to explain enhanced friction on oxidized graphene [8]. However, previous experimental measurements indicate that adhesion decreases slightly due to chemically modifying a surface with hydrogen [10]. This is also exhibited by our simulations, in which the adhesive force on hydrogenated graphene with 10% hydrogen coverage is 49% smaller than that on pristine graphene. Thus adhesion variation is not the dominant reason for the friction increase on hydrogenated graphene.
Then where does the increased friction originate from? For a tip sliding on hydrogenated graphene, the friction force is comprised of two components: the lateral interaction between the tip and hydrogen atoms, and the lateral interaction between the tip and carbon atoms. MD simulation enables us to differentiate the two force components. Figure 3 illustrates the total frictional force and its two components. From the force curves we can see that the lateral interaction between the tip and hydrogen atoms dominates the process and gives rise to the enhanced friction. There is one distinct change after chemically modifying the graphene surface that has not been discussed so far: structural changes due to hydrogenation. Graphene is characterized by its unique two-dimensional structure [24]. When terminated with hydrogen or fluorine, its sp2 carbon bond becomes an sp3 carbon bond and, as a result, the planar graphene sheet is transformed to a three-dimensional tetrahedral structure [9]. Compared to the surface of pristine graphene, the hydrogenated graphene surface is characterized by a much larger atomic-level roughness, as shown in the inset of figure 1. The atomic roughness brings the tip atoms and protruding hydrogen atoms very close, such that they interlock with each other. Consequently, although the adhesive force decreases slightly due to hydrogenation, the lateral force increases substantially due to the lateral interlock. A movie of the sliding process that demonstrates the interlocking of the tip and hydrogen atoms is available in the supplemental materials (available at stacks.iop.org/Nano/24/375701/mmedia).
Figure 3. Friction as a function of sliding distance (solid line) and the contributions from the interactions between hydrogen and the tip (dashed line) and the interactions between carbon and the tip (dotted line).
Download figure:
Standard image High-resolution imageThat atomic-level roughness can substantially increase friction is not a completely new concept. It has support from a similar scenario, friction at atomic step edges, which has received much attention recently. When a nanoscale AFM tip climbs up a step edge, even a single atomic layer high, it requires a much larger lateral force to overcome the interlock between the tip and step edge. This frictional enhancement at step up has been consistently observed on many materials in different experimental environments [25–28], and has been verified by atomistic modeling [29, 35]. Note that the height of the protruding hydrogen atoms in hydrogenated graphene, 0.18 nm, is comparable to that of a single-layer step edge, e.g., the height of one monolayer step edge of NaCl(001) is on the order of 0.28 nm [27]. So a consistent result, friction enhancement, is observed in both cases.
In MD simulation, we can conveniently control the hydrogen coverage on the graphene surface and measure how friction varies with that coverage. In the simulation, we randomly distribute hydrogen atoms on the graphene surface. In order to make the measurement statistically sound, we construct more than five systems with different initial random seeds at each coverage and then use the average friction from these measurements. The results are shown in figure 4, where we observe friction does not monotonically increase with hydrogen coverage. Instead, it reaches a maximum around a coverage of 8%. We propose that this is the result of two competing mechanisms. In previous paragraphs, it was shown that the lateral interlock between the tip and hydrogen atoms causes enhanced friction. The more hydrogen atoms are in contact, the larger the friction becomes if other parameters remain constant. However, when more hydrogen atoms come into the contact, the repulsive force between the tip and hydrogen atoms will push the tip upward, which leads to the weakening of the interlock between the tip and hydrogen atoms. To demonstrate this point, figure 4 shows the gap between the tip and hydrogen atoms increases with hydrogen coverage. This gap is defined as the distance in the normal direction between the bottommost atom in the tip and the average position of hydrogen atoms in contact. The friction peak arises due to the competition between the increase in number of hydrogen atoms in the contact and the weakening of the interlock due to the increase in separation between tip and substrate.
Figure 4. The mean friction as a function of hydrogen coverage (left axis) and the distance between the 3 nm tip and hydrogen atoms with different hydrogen coverage (right axis) with zero externally applied normal load.
Download figure:
Standard image High-resolution imageWe also explore the variation of friction with coverage for model tips with 2 and 4 nm radii and observe that, for all cases, the peak arises between 5 and 10% coverage. This consistency may in part be due to the limited tip size range we can explore: the statistical error due to random distribution of hydrogen atoms on the surface is larger than the friction trends for smaller tips, and the fully atomistic nature of the model and complexity of the empirical potential preclude modeling larger tips. However, the increased friction caused by more hydrogen atoms in the contact area cannot be expected to vary with tip size at the same rate as the decreased friction due to increasing gap. Therefore, it is reasonable to expect that the intersection of these competing effects, the result of which is the friction peak, will vary with tip size for tips much larger or much smaller than those we can access in this study. Other parameters, such as interatomic interaction strength, lattice/orientation of the contacting surfaces, normal load and number of graphene layers may affect the position of the friction peak as well, but they should not affect its existence. Also, it has been shown that hydrogen atoms tend to be attached to graphite in the form of dimers or clusters, especially for low coverage (i.e. ∼1%) cases [30]. This could cause the magnitude and periodicity of measured friction traces (such as those in the inset of figure 2) to differ from those observed here, where the hydrogen atoms are distributed on the surface randomly.
4. Discussion
In this section we consider rigidity as an alternative reason for friction enhancement on hydrogenated graphene. It was mentioned earlier that the sp3 carbon bonds in hydrogenated graphene result in increased surface roughness. This bonding has another effect—it makes the chemically modified graphene much more rigid. That is why diamond with sp3 carbon bonds is the hardest natural material, while the pristine graphene sheet is characterized by the formation of ripples due to its small bending stiffness. It has been proposed that the increase of rigidity, specifically the bending stiffness, might be the reason for friction enhancement on hydrogenated and fluorinated graphene [9, 10]. We will re-examine the proposed mechanism using two methods, the Prandtl–Tomlinson (PT) model and MD simulation.
First, we investigate the effect of bending stiffness on friction using the PT model. The PT model is a reduced-order model and simplifies single asperity friction into one or more point-masses pulled via a harmonic spring along a periodic potential energy profile [31, 32]. Details of the PT model used here can be found in [33]. A harmonic spring in the PT model is used to simulate the effective stiffness in the AFM system. The effective stiffness can be obtained directly from a stick–slip friction curve measured by an AFM as the slope of the force curve during the 'stick' period. The effective stiffness is comprised of a few components, as expressed below [12],
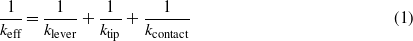
where klever is the stiffness of the micro-cantilever, ktip is the stiffness of the tip, and kcontact is the contact stiffness due to the deformation of the tip apex and substrate. The contact stiffness can be obtained through continuum mechanics as the following [34],
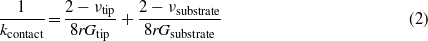
where ν and G are the Poisson ratio and shear modulus, respectively, and r is the radius of the contact area. Kwon et al proposed that lateral force mostly generates out-of-plane bending deformation of the graphene surface. Thus the contact stiffness for graphene or hydrogenated graphene can be expressed as [9]

where kbending is the bending stiffness of the graphene. We observe from equations (1) and (3) that there is a direct relationship between the bending stiffness of the graphene and the effective stiffness in a friction measurement. This means that we can evaluate the effect of bending stiffness by varying the spring stiffness in the PT model. Calculation results obtained from the PT model are illustrated in figure 5. The proposed mechanism, that rigidity is the reason for friction enhancement [9], indicates the friction should increase with an increase of rigidity. However, the opposite trend is observed in the PT model, i.e., friction decreases with an increase of the effective stiffness or rigidity. Also, as shown in the inset of figure 5, although the mean friction varies with the stiffness, the maximum friction remains constant. This differs from the simulation results where, as shown in the inset of figure 2, the maximum friction increases significantly with hydrogenation. The discrepancy highlights the key role of roughness, which is not captured by the PT model. The two inconsistencies mentioned above imply that the change of the bending stiffness cannot solely explain the friction enhancement due to hydrogenation that is observed in experiments and simulations.
Figure 5. Prandtl–Tomlinson model of friction as a function of effective stiffness. The inset shows friction traces corresponding to three different values of the effective stiffness.
Download figure:
Standard image High-resolution imageWe can further investigate the effect of rigidity by directly controlling the flexibility of the graphene in the MD simulation. Specifically, we artificially make the model graphene completely rigid by fixing atoms at their equilibrium crystalline positions. We then measure the friction on rigid graphene and unmodified graphene. As shown in figure 6, a modestly larger friction is obtained on unmodified graphene due to the puckering effect [7, 17]. In other words, increasing rigidity decreases friction.
Figure 6. MD simulation of friction on rigid graphene (dotted line) and unmodified graphene (solid line), respectively. The two simulations are the same except for the rigidity of the substrate.
Download figure:
Standard image High-resolution imageFinally, we show that friction enhancement is a localized event. As illustrated in the inset of figure 7, two strips of the graphene surface are hydrogenated while the other areas remain pristine. We then measure friction across the hydrogenated strips. Figure 7 illustrates that the friction is enhanced only when the tip is in contact with the hydrogenated strips. It is known that a phonon is the collective lattice vibration of atoms, and any local change on the surface can cause the variation of the entire phonon spectrum. If the enhanced friction was due to damping via the flexural phonon associated with rigidity, as proposed in [9], then it would be a non-local process and friction would be modulated everywhere. Although this argument is not conclusive since a localized phonon mode may appear at a defect, in general, the observation that friction modulation occurs only at the hydrogenated strips does not favor the 'rigidity' explanation. Thus, both the PT model and MD simulation indicate that rigidity is not the major reason for friction enhancement on hydrogenated graphene.
Figure 7. MD simulation of friction on strips of hydrogenated graphene as a function of sliding distance. A perspective view of the corresponding graphene substrate is shown in the inset.
Download figure:
Standard image High-resolution image5. Conclusion
In this paper we apply MD simulations to help understand the recent experimental friction measurement on hydrogenated and fluorinated graphene [9, 10]. We demonstrate that it is the atomic roughness due to hydrogenation that gives rise to a dramatic friction enhancement. Adhesion and rigidity as possible reasons for friction enhancement are also discussed but, based on the MD simulation and PT model, they are both excluded as major contributors. Moreover, our results reveal that friction does not monotonically increase with hydrogen coverage; instead a friction peak may exist due to the competition between the increase in number of hydrogen atoms in the contact and the weakening of the interlock due to the increase in separation between tip and substrate. These results indicate that surface modification with hydrogen is a promising method to control friction at the nanoscale in miniaturized mechanical devices.
Acknowledgment
The authors would like to thank the Air Force Office of Sponsored Research for its support through Grant No. FA9550-11-1-0162.







