Abstrect
Block copolymer-based templates can be exploited for the fabrication of ordered arrays of metal nanoparticles (NPs) with a diameter down to a few nanometers. In order to develop this technique on metal oxide substrates, we studied the self-assembly of polymeric templates directly on the HfO2 surface. Using a random copolymer neutralization layer, we obtained an effective HfO2 surface neutralization, while the effects of surface cleaning and annealing temperature were carefully examined. Varying the block copolymer molecular weight, we produced regular nanoporous templates with feature size variable between 10 and 30 nm and a density up to 1.5 × 1011 cm−2. With the adoption of a pattern transfer process, we produced ordered arrays of Pt and Pt/Ti NPs with diameters of 12, 21 and 29 nm and a constant size dispersion (σ) of 2.5 nm. For the smallest template adopted, the NP diameter is significantly lower than the original template dimension. In this specific configuration, the granularity of the deposited film probably influences the pattern transfer process and very small NPs of 12 nm were achieved without a significant broadening of the size distribution.
Export citation and abstract BibTeX RIS
1. Introduction
The controlled generation of metallic nanoparticle (NP) arrays on dielectric films enables applications in different fields of physics and nanotechnology. Owing to the reduced size, noble metal NPs show enhanced catalytic activity [1, 2], while the plasmon-enhanced luminescence associated with metallic NPs offers the possibility to concentrate and manipulate light, enabling a wide variety of applications including light emitting devices, photovoltaic applications and advanced sensing, such as surface-enhanced Raman scattering, localized surface plasmon resonance sensing and second harmonic generation [3–8]. Moreover, noble metal NPs offer an enhanced corrosion resistance to the reactive ion etching process that can be exploited for the patterning of deep features in nanolithographic processes devoted to microelectronic applications [9]. In many cases, the exploitation of metal NPs is closely related to the precise variation of the particle size and density, with the size dispersion being a key parameter. In addition, the ability to define NP arrays with long-range order highly enhances the NPs properties and opens the door to specific applications [10].
Self-assembling materials can be used for the parallel fabrication of ordered arrays of nanoscaled features with controllable size and density along with a limited size dispersion. In particular, block copolymers (BCPs) can be adopted for the synthesis of templates with feature size well below 50 nm, that can be exploited in different nanotechnology fields [11–13].
However, the great majority of BCP applications reported so far involves the self-assembly on top of Si-based substrates. The extension of this tool for the patterning of ordered metal NPs on various dielectric films requires the BCP deposition onto these different substrates without losing control over the BCP domains orientation and lateral ordering.
Among other transition metal oxides, HfO2 gained increasing interest due to its good dielectric properties, high dielectric constant, resistive switching properties and compatibility with the Si-based IC technology [14–16]. The application of BCP to transition metal oxide substrates can open new routes for the nm-scale oxide patterning [17] and the deposition of ordered arrays of nanostructures on top of the oxide surface, to be exploited for the fabrication of various electro-optical devices.
The creation of polymeric templates suitable for pattern transfer requires the formation of domains with perpendicular orientation. To achieve this goal, a neutral surface is required to prevent any preferential wetting of one of the two polymeric blocks. A neutralization layer composed of a random copolymer (RCP) is a viable method on Si-based substrates [18]. Its applicability to HfO2 substrates requires a dedicated investigation in order to achieve an effective surface neutralization.
In the present work, we investigated the surface neutralization of HfO2 films by grafting a poly(styrene-r-methyl methacrylate) P(S-r-MMA) RCP to the surface in order to promote the formation of well-organized BCP nanodomains with a perpendicular direction. The effect of the wet or dry cleaning treatment of the oxide surface was investigated with respect to both surface damaging and the impact on the grafting behaviour. For a grafted RCP thickness above 6 nm, we obtained a complete neutralization of the surface, on top of which we deposited the PS-b-PMMA BCP film. Upon phase separation, the BCP films were used for the creation of nanoporous PS templates with different pores size and spacing by choosing BCPs with various molecular weights (Mw). The produced templates were employed in a pattern transfer process for the formation of ordered arrays of Pt or Pt/Ti metal NPs on top of the HfO2 oxide. An acid solution and a solvent soak were alternately applied for the lifting-off of the PS template, and their impact on the final pattern transfer is herein discussed. The reported process allowed the fabrication of arrays of metal NPs arranged in a hexagonal periodic distribution with diameter down to 12 nm and a good control over the diameter distribution.
2. Experimental
2.1. Substrates preparation and cleaning
HfO2 films of 3 and 6 nm were deposited by atomic layer deposition (ALD) in a Savannah reactor (Cambridge Nanotech) [19, 20] on TiN films and on Si substrates with native oxide. The as deposited HfO2 films of 3 nm were found to be amorphous by x-ray diffraction measurements, while the 6 nm films are partially crystalline. In order to fully crystallize the 6 nm films, samples were annealed in a rapid thermal processing (RTP) machine at 500 °C for 60 s in N2 ambient.
The HfO2 surface was cleaned either in piranha solution (H2SO4/H2O2 with 3/1 volume ration at 80 °C for 40 min) or with oxygen plasma (40 W for 2 min). Samples were then sonicated in an isopropanol bath prior to polymer solutions spinning for cleaning from particles contamination.
2.2. Surface functionalization
A brush layer of hydroxyl-terminated P(S-r-MMA) RCP with 0.62 styrene fraction, 14.5 Kg mol−1 molecular weight and polydispersivity index (PDI) 1.25 was deposited on the HfO2 surface by spinning a solution of 18 mg in 2 mL of toluene at 3000 rpm for 30 s. The surface neutralization was achieved by grafting the P(S-r-MMA) polymer chains to the hydroxyl groups present on the oxide surface by thermal treatment in an RTP machine in N2 atmosphere for 10 min at temperatures between 230 and 310 °C. The non-grafted polymer chains were afterward removed by washing in toluene for 5 min. The thickness of the grafted RCP was measured by means of spectroscopic ellipsometry (M-200U, J A Wollam Co. Inc.).
2.3. Block copolymer template
Asymmetric PS-b-PMMA BCPs with styrene fraction ∼0.7 (Polymer Source Inc.) in toluene solution (18 mg in 2 mL) were spun on the RCP-treated surface at 3000 rpm for 30 s, obtaining a polymer thickness of ∼30 nm. The nanoscale phase separation was induced with an RTP treatment at 250 °C in N2 atmosphere, with process time varying from 5 to 15 min. After annealing, a regular array of hexagonally packed PMMA cylinders perpendicular to the surface embedded in a PS matrix was obtained. In order to vary the size and spacing of the nanodomains, various Mw were adopted: 54 Kg mol−1 (PDI 1.07), 67 Kg mol−1 (PDI 1.09) and 102 Kg mol−1 (PDI 1.08). The final nanoporous template used as a soft mask for the BCP-based lithography was obtained by selectively removing the PMMA component by degrading the polymer chains under UV light exposure (λ = 253.7 nm; 5 mW cm−2), followed by soaking in acetic acid for 8 min, rinsing in deionized water and drying under N2 flow. The pores were finally opened and the RCP on the bottom was removed by exposure to oxygen plasma (40 W), obtaining a nanoporous PS template.
2.4. Metal deposition and patterning
Thin films of 5 nm of Pt or 6 nm Pt/4 nm Ti were physically evaporated through the nanoporous PS templates. Electron-beam evaporation was the deposition method of choice since it allows a directional covering of the template walls, giving better results for pattern transfer if compared to other techniques (i.e. sputtering, CVD, etc). The PS template together with the overlying excess metal were afterward removed either in piranha solution [21] or in a sonicated bath of warm toluene [12, 13, 22].
2.5. Samples imaging and data analysis
Images of the nanoporous PS templates and metal dot arrays were acquired using a field emission scanning electron microscope (Supra 40, Carl Zeiss AG) operated at the acceleration voltage of 15 KV and 4 mm working distance using the in-lens detector.
The AFM images were acquired in tapping mode using a Dimension Edge instrument (Bruker) equipped with a topography probe (PPP-NCHR, Nanosensors).
The determination of the size of the characteristic features in the polymeric templates and in the patterned samples was carried out by software analysis applying a threshold mask to the SEM images, then the equivalent radius was extracted using the grain distribution function of Gwyddion software. The reported mean values and standard deviations were extracted by a Gaussian fit to the data. At least four SEM images of 1.125 × 0.774 μm were used for statistical data analysis for each sample.
3. Results and discussion
3.1. Surface cleaning and neutralization
The first step in the lithographic process is the substrate cleaning. This step is required to remove contaminants like carbon impurities and particles, however it can have an impact also on the surface energy (i.e. change the wettability of the surface) [23] and occasionally affect the substrate properties. Piranha solution is often applied for the removal of carbon contaminants from SiO2 substrates and is widely applied prior to RCP and BCP spinning [21, 22, 24, 25]. The effect of the piranha surface treatment in producing a highly hydrophilic surface was reported for silica and borofloat glass substrates [26–28], greatly improving the grafting of the RCP chains. This wet cleaning is however highly aggressive and entails a very poor materials compatibility, limiting its applicability for transition metal oxides. For the HfO2 material, we found that the as deposited amorphous film is completely removed by a few minutes of immersion in piranha solution. If however the sample is annealed at a high enough temperature, the HfO2 film crystallizes and only 0.5 nm of the original layer are removed after 40 min in piranha solution, a thickness compatible with the removal of the carbon contaminants. A deeper inspection however reveals the appearance of holes and cracks in the treated film, indicating that a surface damage occurred. These modifications are clearly visible in the SEM pictures of the HfO2 surface and can be identified even in AFM topographic maps of the treated surface (figure 1).
Figure 1. SEM plane views of the annealed HfO2 surface before (a) and after (b) cleaning in piranha solution. Some holes and cracks appear on the surface. AFM surface topography before (c) and after (d) cleaning also reveals the appearance of surface damages.
Download figure:
Standard image High-resolution imageIn order to avoid any damage of the HfO2 film, an alternative route for the surface cleaning from carbon contaminants can be a selective dry etch like oxygen plasma [29]. For an oxygen plasma treatment of 120 s at 40 W, no detectable HfO2 film thickness variation was found by spectroscopic ellipsometry, while from SEM and AFM analysis the appearance of major surface topography modifications was excluded for both crystalline and amorphous films.
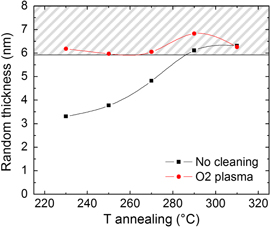
Furthermore, a relevant question is how the plasma treatment affects the wettability of the HfO2 oxide and creates bonding sites for the RCP chains grafting to the oxide surface, i.e. how the hydroxyl groups are modified during the plasma treatment. In figure 2, the grafted RCP thickness is reported as a function of the annealing temperature for the O2 plasma-treated samples, with the uncleaned samples inserted for reference. It is worth noting that the thickness variation among repeated experiments lies within 0.5 nm. For temperatures lower than 290 °C, the O2 plasma highly enhances the grafting behaviour, resulting in a flat trend as a function of temperature even at 230 °C, allowing to obtain an optimal surface neutralization at lower temperature. On the contrary, the untreated surface requires a temperature higher than 290 °C for the complete neutralization to occur, showing a monotonous increase of the grafted thickness for increasing annealing temperatures, meaning that a higher kinetic energy is required for the RCP chains to find available OH sites on the surface. At 310 °C, both treated and untreated samples reach the same saturation thickness, indicating that all the available OH sites are grafted to RCP chains. Since the saturation level is equal for both sample series, the different dependence on temperature is to be related to more reactive hydroxyl groups formed after O2 plasma exposure, rather than to an increase of the number of hydroxyl groups.
Figure 2. Thickness of the grafted RCP layer as a function of annealing temperature. While the uncleaned samples show a monotonous increase of the grafted polymer thickness for increrasing temperatures, the samples treated with oxygen plasma have a nearly flat trend and lie in the full neutralization region (highlighted with grey stripes) for all the inspected temperatures.
Download figure:
Standard image High-resolution imageIn summary, for the ALD-grown HfO2, improved results in terms of RCP grafting are obtained using the dry O2 plasma cleaning of the surface, which in turn offers the advantage of a broader process compatibility in comparison with the wet piranha cleaning.
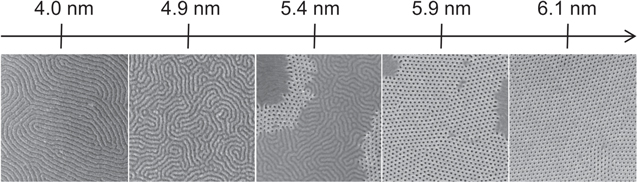
In order to investigate the effect of the grafted RCP thickness on the effective surface neutralization, we analysed the orientation evolution of the cylindrical nanodomains in BCP layers deposited over various RCP thicknesses (figure 3). For a thickness ≤4 nm, cylinders completely parallel to the surface appear after nanophase separation throughout the sample surface. Increasing the RCP thickness, the perpendicular orientation starts to nucleate and above 5 nm areas with perpendicular orientation appear. The relative coverage of the areas with perpendicular orientation increases with increasing RCP thickness and for a thickness ≥6 nm the cylinders are perfectly oriented perpendicularly to the surface. This effective surface neutralization can be achieved for a RCP annealing temperature ≥290 °C for the uncleaned samples or throughout the whole tested temperature range for the O2 plasma-cleaned samples.
Figure 3. Evolution of the BCP morphology as a function of the RCP layer thickness. Above 6 nm, a complete surface neutralization can be obtained, leading to a perpendicular cylinders morphology. The edge of the SEM pictures corresponds to 1 μm.
Download figure:
Standard image High-resolution imageThe maximum RCP thickness achievable, together with the value above which a perfect neutralization is obtained, is highly dependent on the RCP molecular weight. In this case, a Mw of 14.5 Kg mol−1 was adopted, obtaining a saturation thickness slightly lower than what is obtained on SiO2 [25, 30], suggesting that a lower density of available hydroxyl groups is present on the HfO2 surface, even after surface treatment. These results however demonstrate the possibility to obtain a complete surface neutralization and thus a perpendicular BCP nanodomains orientation on HfO2 substrates. The same RCP grafted thickness and an effective surface neutralization above 6 nm was obtained on both amorphous (as deposited) and crystalline (annealed) HfO2 films of 6 nm and on amorphous films of 3 nm (as deposited), indicating that the hydroxyl groups type and content do not vary significantly during the ALD growth and are not changed by the oxide post-deposition annealing treatment.
3.2. Template formation
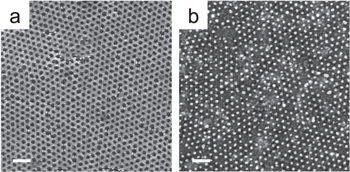
After effective surface neutralization by RCP grafting to the HfO2 surface, BCP thin films of ∼30 nm were spun on the neutralized surface. Different Mw were adopted, allowing the formation of templates with different pores density and diameter [25, 31]. The nanoscale phase separation was promoted by thermal annealing in an RTP machine, which allows to reduce the processing time to a few minutes [32]. An annealing time of 5 min was sufficient to obtain a well ordered periodic structure for all the considered BCPs, while the correlation length, defined as the mean distance over which the periodic distribution is conserved, greatly varies for the different Mw and also depends on the annealing time and temperature [33]. The pores density attainable with the different Mw used is reported in table 1, while in figure 4 an SEM plane view of the three nanoporous templates is reported.
Table 1. Density of the pores obtained for three different BCP molecular weights. The uncertainties correspond to the standard deviations.
| Mw (g mol−1) | 54 K | 67 K | 102 K |
|---|---|---|---|
| Pore density (×1011 cm−2) | 1.520 ± 0.014 | 1.015 ± 0.015 | 0.520 ± 0.010 |
Figure 4. SEM plane views of the nanoporous PS templates obtained after 30 s of O2 plasma exposure from BCP with Mw of (a) 54 Kg mol−1, (b) 67 Kg mol−1 and (c) 102 Kg mol−1. (Scale bars: 100 nm).
Download figure:
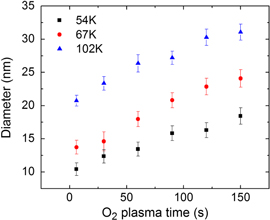
Standard image High-resolution imageWhile the density of the pores is fixed for a particular Mw, the mean pore diameter strongly depends on the O2 plasma time that is necessary to completely open the pores and remove the RCP from the bottom, uncovering the oxide surface. Indeed, after the PMMA component has been degraded and removed, an exposure to O2 plasma isotropically erodes the remaining PS matrix, enlarging the pores in a mostly linear fashion as a function of time (figure 5). The error bars of figure 5 depict the standard deviation of the pores diameter as derived from multiple SEM images. Even if the pores are enlarged by nearly 10 nm after 150 s, the diameters dispersion only slightly increases and σ remains below ±1.3 nm, meaning that the control over the pores size distribution is not worsened by the O2 plasma exposure.
Figure 5. Diameter of the pores in the BCP-formed PS template for increasing oxygen plasma time (from 6 to 150 s) for three different BCP molecular weights.
Download figure:
Standard image High-resolution imageFigure 5 also reports a substantial diameter overlap for different Mw obtained by adjusting the O2 plasma time. This property potentially allows a decoupling between the defined features density and their dimension. However, for an RCP thickness of ∼6 nm, a minimum plasma time of 150 s is required to remove the bottom-lying RCP and completely open the pores in order to perform a pattern transfer using a lift-off process. As an example, in figure 6 an incomplete pattern transfer is reported for samples exposed to 120 s of O2 plasma.
Figure 6. Lift-off process for 5 nm of Pt performed in piranha solution after 150 s (a) and 120 s (b) of O2 plasma on templates formed from a 67 Kg mol−1 BCP. A poor pattern reproduction is obtained after 120 s, indicating an incomplete opening of the pores. (Scale bars: 100 nm).
Download figure:
Standard image High-resolution image3.3. Metal deposition and patterning
Platinum is widely employed as electrode material in nanoscaled systems, as well as active material in sensors and catalytic applications [34], where its nanostructuration is required. Here we employ an electron-beam physical deposition method to deposit Pt thin films with a thickness of 5 nm over the BCP template, in order to obtain ordered arrays of nanosized Pt dots over the HfO2 surface. The deposited thickness respects the rule of thumb of ≤1/3 of the polymer template thickness, necessary for the fulfilment of the lift-off process. If a higher metal thickness is required, thicker template masks can be deposited by either lowering the spinning velocity or depositing a denser polymer solution [33].
The electron beam evaporation method offers the advantage of a low chamber pressure (∼10−6 mbar) and a less energetic deposition if compared for example with the sputtering method, allowing a good separation between the metal covering the template and the metal within the pores, resulting in an easier template removal.
After the metal deposition, the template mask should be dissolved and removed together with the excess metal on top. Two wet methods (acid or solvent) were applied. The immersion of the sample in piranha solution rapidly removes the polymeric component, resulting in big areas developed in a short time. However, the etch rate is so high that the process can be difficultly controlled. Additionally, only a few materials have a sufficiently high etch selectivity to be patterned with this etchant [35]. Another concern is the surface damage that can be produced even on a crystallized HfO2 substrate, as reported in figure 1.
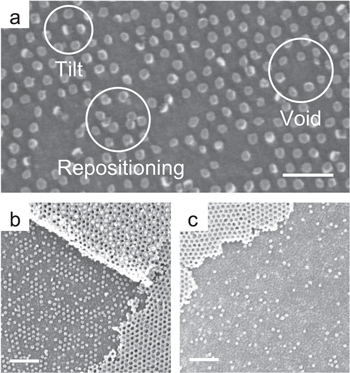
Platinum is among the few metals compatible with piranha. However, several flaws manifested with increasing processing time. In figure 7, the same sample analysed after 5 and 25 min of piranha soak shows a clear loss of the patterned particles and a deterioration of the periodic distribution for increasing processing time. The type of the generated flaws, including repositioning of the metallic dots and tilting, indicates a poor Pt surface adhesion.
Figure 7. Flaws arising after patterning of a 5 nm Pt film by piranha wet etch for 10 min (a). Lift-off process tested after 5 min (b) and 25 min (c). A clear deterioration of the transferred pattern can be observed with increasing time. (Scale bars: 100 nm).
Download figure:
Standard image High-resolution imageAn alternative route to the piranha etch can be a selective solvent for the polymeric template, an approach that brings a clear advantage in terms of materials compatibility. Toluene is a PS selective solvent, however during the O2 plasma treatment a partial cross-linking of the polymer chains occurs. Starting from 30 s of plasma time, the solubility is greatly decreased. The consequence is that long baths in warm toluene are required, while a low power sonication should be added to break the metallic film, aiding its removal. With this method, areas of the sample with lateral dimension of the order of hundreds of micrometers were effectively patterned, but a whole template removal is difficult to achieve unless very long times are applied. The lift-off performed in toluene allowed a better pattern transfer, with the disappearing of flaws caused by repositioning of the metal dots (figure 8). Nevertheless, the long wet process and the addition of sonication, even at low power, caused the loss of some of the patterned features in the periodic matrix.
Figure 8. Patterning of a 5 nm Pt film by lift-off in a warm toluene bath. (a) The residual metal covering the PS template shows some cracks due to sonication. (b) The template obtained from the 67 Kg mol−1 BCP is well reproduced, but some patterned features are missing. (Scale bars: 100 nm).
Download figure:
Standard image High-resolution imageFor application purposes, platinum is known to have a poor surface adhesion on many materials, including transition metal oxides. The addition of an interlayer of titanium greatly improves the pattern adhesion [36, 37]. In figure 9, the pattern transfer of Pt/Ti metal dots by lift-off in toluene is reported for the different BCP templates adopted.
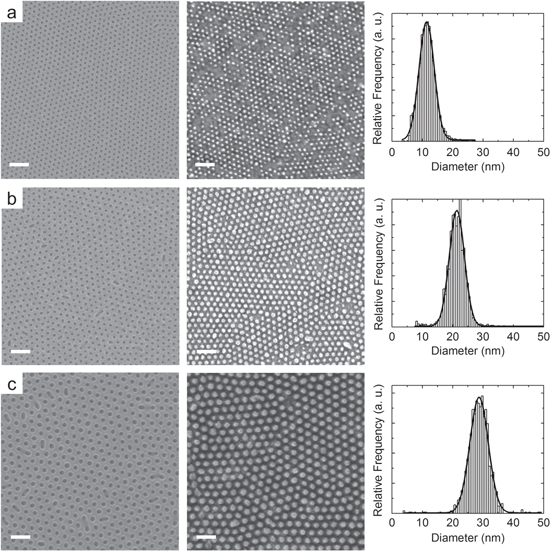
Figure 9. BCP-based templates originated from polymers with Mw of 54 Kg mol−1 (a), 67 Kg mol−1 (b) and 102 Kg mol−1 (c) and the relative Pt/Ti dots obtained by lift-off process. On the right, statistical distributions of the dot diameters. (Scale bars: 100 nm).
Download figure:
Standard image High-resolution imageComparing the PS nanoporous template with the obtained metal NPs, a perfect pattern transfer can be found for the 67 and 102 Kg mol−1 BCPs, with flaws only coming from the original template, mainly in correspondence with grain boundaries. The 54 Kg mol−1 BCP constitutes a particular case. The small dimension of the pores and their close vicinity establishes a nearly continuous metallic film after deposition. This adds a difficulty in the template removal, requiring longer processing times and a higher sonication power, leading to a complete loss of the patterned features. In order to degrade the polymer in a faster time, we adopted a short dip in piranha solution (5 s) before soaking in toluene. It is worth to note that this very short dip does not impact significantly on the underlying HfO2 surface. The result is reported in figure 9(a). Contrary to the templates with higher Mw, more deficiencies can be identified, which can be either related to a not complete opening of the pores or to the fast piranha dip required in this case.
The pores diameters for the three adopted templates and the relative dots diameters after pattern transfer are summarized in table 2. For the 67 and 102 Kg mol−1, the resulting metal dots are about 2 nm smaller than the original template holes, a value still compatible within the uncertainties. The slightly smaller dimension can be related to a partial covering of the template walls. For the 54 Kg mol−1, the discrepancy is higher, about 6 nm. Metal NPs with a diameter of only 12 nm were obtained in this case, while the width of the Gaussian distribution is similar to that obtained with the wider templates.
Table 2. Pore diameters after 150 s oxygen plasma treatment and relative Pt/Ti dot diameters after pattern transfer. The uncertainties correspond to the standard deviations.
| Mw (g mol−1) | 54 K | 67 K | 102 K |
|---|---|---|---|
| Pores diameter | 18.4 ± 1.2 | 22.9 ± 1.2 | 31.1 ± 1.2 |
| Dots diameter | 12.0 ± 2.5 | 21.4 ± 2.5 | 28.8 ± 2.7 |
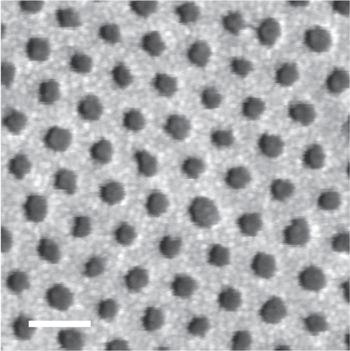
The experimental results suggest that the process of pattern transfer operated with the smallest template reaches a limit beyond which the final result changes significantly. A plausible explanation is that when the dimension of the pores is approaching the dimension of the grains composing the Pt film, the deposited film can no longer be seen as continuous. At this size, the granularity of the patterned film could influence the result of the pattern transfer technique [38]. Indeed, SEM images of the deposited Pt/Ti film evidence the presence of grains with an average dimension of 6–7 nm (figure 10). The result is that NPs of only 12 nm were produced, without losing control over the particles size distribution.
Figure 10. Pt/Ti film deposited over the PS nanoporous template. Grains of about 6–7 nm are distinguishable in the metal film. (Scale bar: 50 nm).
Download figure:
Standard image High-resolution imageThe proposed fabrication method on top of HfO2 films can be easily extended to the patterning of other materials with the same control over the defined features size and density, which are mainly determined by the self-assembled polymeric templates adopted. The final dimension of the NPs obtained with the smaller templates is however likely to depend also on the nanostructure characteristics of the deposited film, which can result in a particle size smaller than the original template as in the present case.
Different fabrication methods can be applied in order to obtain noble metal NPs with controlled size and density. For instance, the deposition of thin (<50 nm) Pt or Au films followed by heat treatment leads to the agglomeration of the metal film in NPs with controllable size by solid-state dewetting [39–41]. In comparison with the BCP-based process, this method often requires higher processing temperatures and results in a worse control over the NPs size distribution. Most importantly, if a periodic pattern is required for the NPs matrix, pre-patterned substrates are needed, dramatically increasing the complexity of the process [42]. Conversely, the metal deposition assisted by colloidal PS particles [5] and the block copolymer micelle lithography [4] allow the generation of periodically arranged noble metal NPs over large areas, but they allow a lower control over the quality of the obtained metal NPs and the adoption of an aqueous solution strongly limits the fields of application. Alternatively, by tailoring the interaction between the NPs and the BCP blocks in block copolymer–NP composites, it is possible to direct the location of pre-synthesized NPs exploiting BCP self-assembly [43–45]. In conclusion, the advantage of our method consists in a very uniform control over the NPs size distribution for different dimensions, together with the possibility to reach extremely high densities.
4. Conclusions
Metal NPs with periodic distribution and controlled size and density were fabricated on top of a transition metal oxide film by pattern transfer with the assistance of BCP-based templates.
This work demonstrates the feasibility of the PS-b-PMMA block copolymer self-assembly with perpendicular orientation of the cylindrical nanodomains on top of the HfO2 surface, with the adoption of a RCP neutralization layer. The effective surface neutralization was found to be strongly dependent on the surface cleaning procedure. The oxygen plasma treatment affects the wettability of the HfO2 surface, lowering the process temperature at which a complete surface neutralization can be obtained.
Nanoporous templates obtained from BCPs with three different molecular weights (54, 67 and 102 Kg mol−1) were produced. In this way, a good control over the template features size and density was achieved, obtaining a density between 0.5 and 1.5 × 1011 cm−2.
The pattern transfer was performed using either an acid etch or a solvent soak. While the acid etch brings the advantage of a very fast process, a better template reproduction was obtained with the solvent treatment, with the advantage of a broader metal compatibility and the avoidance of any oxide surface damage. The cross-linking of the polymer template during the pores opening however hinders the large-scale applicability of the solvent treatment, in particular for the smallest template used (54 Kg mol−1), and further study is required.
Both Pt and Pt/Ti NPs arranged in a hexagonal periodic distribution over the HfO2 surface were obtained, with a diameter varying from 12 to 29 nm and a standard deviation around 2.5 nm. For the smallest template adopted, the final particle size is significantly smaller than the original template pore dimension. A possible explanation can be found in the granularity of the deposited metal film, which starts to be comparable with the dimension of the apertures in the template. For this reason, Pt/Ti NPs well-controlled in size and with a diameter of only 12 nm were achieved.
Acknowledgments
The authors thank Michele Laus and Katia Sparnacci (DISIT, Università del Piemonte Orientale) for providing the random copolymers and Elena Cianci (MDM, IMM-CNR) for the HfO2 ALD depositions.