Abstract
In order to utilize the superb electronic properties of graphene in future electronic nano-devices, a dependable means of controlling the transport properties of its Dirac electrons has to be devised by forming a tunable band gap. We report on the ion-induced modification of the electronic properties of single-layer graphene (SLG) grown on a SiC(0001) substrate by doping low-energy (5 eV) Li+ ions. We find the opening of a sizable and tunable band gap up to 0.85 eV, which depends on the Li+ ion dose as well as the following thermal treatment, and is the largest band gap in the π-band of SLG by any means reported so far. Our Li 1s core-level data together with the valence band suggest that Li+ ions do not intercalate below the topmost graphene layer, but cause a significant charge asymmetry between the carbon sublattices of SLG to drive the opening of the band gap. We thus provide a route to producing a tunable graphene band gap by doping Li+ ions, which may play a pivotal role in the utilization of graphene in future graphene-based electronic nano-devices.
Export citation and abstract BibTeX RIS
1. Introduction
Graphene, a representative two-dimensional (2D) material with a single atomic layer of carbon atoms, has attracted extensive research efforts mainly due to its exotic properties stemming from its relativistic Dirac fermions with linear energy–momentum dispersion (Dirac π-band) [1–4]. Despite the outstanding properties of SLG, which have been well characterized to date, its semi-metallic nature showing no band gap (Eg) in the π-band hinders its application in electronic circuit elements for graphene-based electronic nano-devices. Several schemes, therefore, have been adopted to form a band gap in graphene by, for example, hole- or molecular-doping [5, 6], electron confinement with quasi-one-dimensional graphene nanoribbons [7, 8], a gate voltage applied perpendicularly to bilayer graphene [9], and chemical functionalization by adsorbing external atoms or molecules on SLG [10, 11]. The opening of a band gap by functionalizing graphene with atoms and molecules, in particular, has been utilized efficiently to induce symmetry-breaking in the charge distribution between the A and B carbon sublattices of a hexagonal unit cell of SLG [12–15]. The degradation of the unique Dirac nature of graphene by adsorbates, however, appears to be a serious drawback for industrial applications, and needs to be tackled. In comparison, the doping of Na+ or Cs+ ions on graphene has recently been reported to open a band gap without causing serious deterioration of the linear π-band [16, 17].
Here, we report on another unique feature of the Dirac band of SLG induced by Li+ ions of very low energy (5 eV), motivated by the unique role of lithium atoms and ions in driving the exotic properties of SLG, such as 2D superconductivity [18, 19] and rechargeable Li-ion batteries [20]. Despite the extensive theoretical studies on Li or Li+ adsorbed SLG to induce a band gap [21–24], no experimental realization has been reported so far. We now report that the doping of Li+ ions on SLG does indeed open a band gap whose size can be adjusted artificially in proportion to the Li+ ion coverage (θ) followed by an annealing procedure up to Eg = 0.85 eV. We notice minimal adverse effects on the Dirac nature of the π-band by doping Li+ ions on SLG. Our photoemission data (valence and core levels) suggests that the opening of a band gap by Li+ ions is driven essentially by the Li+-induced broken symmetry in the charge distribution among carbon atoms. This way of opening a band gap by doping low-energy Li+ ions thus turns out to be quite an effective route for forming a tunable band gap, without damaging the Dirac nature of SLG.
2. Experimental methods
Our SLG sample was epitaxially grown on a Si-terminated n-type 6H-SiC(0001) wafer purchased from SiCrystal AG with chemical and mechanical polishing done on the silicon face. The growth of homogeneous SLG on the 6H-SiC(0001) substrate was performed by annealing the sample in situ resistively with the annealing temperature (Ta) in the range of 1150 °C ≤ Ta ≤ 1200 °C in an ultrahigh vacuum environment with a base pressure of low 10−10 Torr, as reported earlier [25–27]. The formation of SLG was examined first by observing a series of low electron energy diffraction (LEED) patterns; a Si-rich 3 × 3 phase appears initially upon annealing at temperature Ta = 850 °C under Si flux for an hour, and then a √3 × √3-R30° phase with Ta = 950 °C, which turns into a 6√3 × 6√3-R30° phase—a precursor phase of the buffer layer—before forming an SLG after continued annealing at Ta ∼ 1200 °C, with its characteristic LEED spots. The SLG thus formed shows a well-defined linear π-band near the K-point of the Brillouin zone (BZ) obtained by using angle-resolved photoemission spectroscopy (ARPES). We have examined the changes in the π-band and in the Li 1s core level using synchrotron photons of energy 34 eV and 68 eV, respectively, at the 4A2 beamline of the Pohang Accelerator Laboratory in Korea. The photoemission chamber was equipped with a hemispherical electron analyzer VG-Scienta R4000 for the valence band, and a Scienta SES 2002 analyzer for the core levels, with an energy resolution less than 100 meV. The Li+ ions were deposited by using a low-energy alkali metal ion gun (Kimball Physics Inc.). The dose of Li+ ions on the SLG was calibrated by measuring the work function change versus the ion dose curve assuming one monolayer (1.0 ML) at saturation (not shown). We determined the temperature of the sample by using an optical pyrometer, and all the sample photoemission measurements were made at a temperature of 90 K.
3. Results and discussion
In figures 1(a)–(e), we present the gradual changes of the π-band with an increasing dose θ of Li+ ions on SLG accompanied by successive thermal treatments. The π-band from clean SLG in figure 1(a) shows the Dirac point (DP, yellow spot) at 0.42 eV below the Fermi energy (EF) due to the charge transfer (∼1.3 × 1013 cm−2) from the substrate [28, 29]. In figures 1(b)–(d), one quickly notices the opening of a band gap with an increasing dose of Li+ ions up to Eg = 0.5 eV for θ = 1.0 ML, while the DP shifts down away from EF. It is interesting to note the n-doping induced by low-energy (5 eV) Li+ ions, which is in sharp contrast with the p-doping of about 0.2 eV observed in high-energy ion-doped SLG samples, i.e., Ar+ (500 eV) [30], Na+ (100 eV) [16], and Cs+ (100 eV) [17], where some ion-induced defects are present. Such different doping effects, depending on the energy of doped ions, may arise from different Bader's conditions—i.e., no gradient in the charge density ρ along the surface normal ∇ρ = 0 [31]. To establish a new Bader's condition for each kind of ion, the resulting charge distribution in SLG produces a different charge asymmetry among the carbon atoms to cause a different size of band gap opening. The sensitive nature of Li+-induced band gap openings compared to that of other ions, for example, Cs+ where the band gap begins to open at θ = 1.0 ML [17], may offer technological merit in functionalizing graphene for industrial applications.
Figure 1. Changes of the π-band induced by Li+ ions as a function of θ and thermal annealing. The π-band obtained from (a) SLG and (b), (c), (d) after doping Li+ ions of θ = 0.3 ML, 0.6 ML, 1.0 ML, and (e) the annealed band in (d) at 300 °C, respectively. (f) MDC (blue curves) and EDC (red curve) around the K-point of the band (e). Eg is found to increase from 0.5 eV to 0.85 eV upon annealing. (g) Change of in-gap intensity around the DP with θ. Inset shows the change of the peak value. (h) Changes of Eg and carrier concentration n with increasing θ and thermal annealing.
Download figure:
Standard image High-resolution imageThe Li+-induced band gap increases gradually with increasing θ up to θ = 1.0 ML, reaching Eg = 0.5 eV with an increased n-type doping effect, as seen in figure 1(d). In contrast, we notice no band gap produced when neutral Li atoms are adsorbed on SLG, where the Li atoms sit at the symmetric hollow sites of the carbon hexagon of the graphene surface [32]. It is interesting to note that the π-band becomes diffused by the random spatial distribution of neutral Li atoms on SLG, while it remains relatively sharp with the doping of Li+ ions. The Fermi velocity (vF) estimated from the slopes near the DP [33] of the Li+-doped SLG, vF = 0.95 × 106 m s−1, appears to be significantly greater than the value 0.60 × 106 m s−1 of the Na+-doped SLG, but nearly the same as that (1.0 × 106 m s−1) of pristine SLG. This indicates that the π-band sustains its Dirac nature by the doping of low-energy (5 eV) Li+ ions, but is not quite able to do so with high-energy (100 eV) Na+ ions [16].
Another interesting observation depicted in figure 1 is the annealing effect. One finds almost no effect upon the annealing of the Li+-doped SLG at 200 °C in figure 1(d), where the DP remains almost unchanged (not shown), though a significant change in the π-band occurs when it is annealed at 300 °C (figure 1(e)) where both the size of the band gap and the n-doping effect increase considerably. The much enhanced thermal diffusion of Li+ ions at 300 °C, as suggested by a recent theory [34, 35], may increase the occupation of asymmetric binding sites for the Li+ ions, which should then enhance the charge asymmetry among carbon atoms to increase the size of the band gap. After annealing at 300 °C, we find a band gap of Eg = 0.85 eV, as revealed by the energy distribution curve (EDC) at the K-point in figure 1(f), and also by the reduced intensity at the DP in the momentum distribution curves (MDCs) shown in figure 1(g). These changes described above, both in the size of the band gap and the doping level, or carrier concentration n, calculated from the estimated vF with increasing θ and Ta, are summarized in figure 1(h).
We note that the size of the band gap Eg = 0.85 eV is the largest band gap of all the SLG functionalized with doping atoms or ions reported so far—including O (0.45 eV) [13], H (0.45 eV) [14], Na (0.74 eV) [36, 37], Cu (0.36 eV) [38], Au (0.25 eV) [39], Na+ (0.7 eV) [16] and Cs+ (0.68 eV) [17]. The notably reduced intensity, both in the EDCs and MDCs near the DP in figures 1(f)–(g) (though its origin remains controversial [28, 40]), clearly demonstrates the presence of a band gap induced by Li+ ions. This suggests that Li+-doped SLG may be utilized more efficiently as a circuit material in future graphene-based electronic devices with more minimal adverse effects from either the in-gap states [28] or many-body interactions [40] than the other functionalized SLG reported so far with no such features [13, 36, 37].
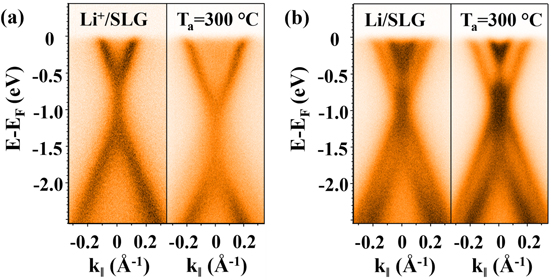
As briefly mentioned earlier, the difference in the changes of the π-band induced by the Li+ ions and by the neutral Li atoms is quite different, see figure 2. One finds a single π-band with a band gap in figure 2(a) whose size increases upon annealing for Li+-doped SLG, while the two π-bands with no band gap in figure 2(b) reflect the presence of bilayer graphene for the neutral Li-adsorbed SLG [19, 41–44]. Such a distinct difference provides a clue to understanding the driving force of the Li+-induced band gap. No band gap with the neutral Li atoms sitting at the symmetric hollow sites, as well as the presence of a Li+-induced band gap, immediately suggest that the Li+ ions have to occupy asymmetric binding sites to cause a certain kind of charge asymmetry between the two carbon sublattices of graphene.
Figure 2. Comparison of the π-band modified by doping Li+ ions (θ = 1.0 ML) in (a) and adsorbing neutral Li atoms in (b). One finds a single π-band with a band gap for Li+-doped SLG and two π-bands with no band gap for the Li-adsorbed SLG. The annealing at 300 °C increases the band gap for the Li+-doped SLG.
Download figure:
Standard image High-resolution imageOne further finds that Li atoms intercalate below the buffer layer to form bilayer graphene as for other neutral atoms [45–49], while there is no such intercalation for Li+ ions, as suggested by our core-level data discussed below. The gradual changes of the Li 1s core level with increasing θ, and also with the consecutive annealing process presented in figure 3, provide further insight that accounts for the corresponding changes in the valence band shown in figure 1.
Figure 3. Changes in the Li 1s core level spectra with increasing θ and thermal annealing. (a) θ = 0.3 ML, (b) 0.6 ML, (c) 1.0 ML, and (d) after annealing (c) at Ta = 300 °C. The four components, D (red), H (blue), T/B (green), and AH (pink) represent the Li+ ion occupying defect, symmetric hollow, top/bridge, and asymmetric hollow sites, respectively, as depicted schematically in (e). (f) Changes of the relative abundance of each component with θ.
Download figure:
Standard image High-resolution imageOne notices characteristic changes in figures 3(a)–(c) induced by the Li+ ions for θ = 0.3, 0.6, 1.0 ML, and the annealing effect shown in figure 3(d) at temperature Ta = 300 °C, respectively. We have fitted the Li 1s core levels with four components; D, H, T/B, and AH with binding energies Eb = 56.6 eV, 55.5 eV, 55.1 eV, and 54.4 eV, respectively. These components denote the Li+ ions sitting on the defect (red), hollow (blue), top/bridge (green), and asymmetric hollow (pink) of the carbon hexagon, respectively, as depicted in figure 3(e). The component D represents the Li atoms sitting at the sites nearby the intrinsic defects [43]. Our measured binding energy difference between the H and T/B sites ΔEb = 0.40 eV matches quite well with the theoretical value ΔEa = 0.34 eV for the difference in adsorption energies for these sites [32]. We have ignored the difference between the top and bridge sites and considered them as one component, since the difference in adsorption energy between these sites is estimated to be negligibly smaller than 0.02 eV [32]. Interestingly enough, the AH site is found to have the lowest binding energy among all the possible binding sites for Li+ ions, suggesting that the Li+ ions sit at the energetically less stable sites. The changes of relative occupancy of these binding sites represented by their respective spectral intensities are summarized in figure 3(f) where the stable H sites dominate over the other sites at all ion coverages.
However, one notes that the relative abundance of AH increases rapidly at the expense of the decreasing H site upon annealing at 300 °C, when the band gap increases to its maximum value of Eg = 0.85 eV (figure 1(e)). One of the plausible scenarios that would account for this rather unusual situation, where Li+ ions occupy energetically the least favored AH binding sites upon annealing, is to consider the Coulomb repulsions among Li+ ions that may push the Li+ ions slightly away from the H sites and into the AH sites. As the number of Li+ ions at the AH sites increases as a result of mutual repulsions, the charge asymmetry among the carbon atoms develops further to increase the size of the band gap already produced with the smaller number of Li+ ions at the AH sites before annealing. This scenario may consistently explain the changes in the π-band in figure 1 and also in the Li 1s core level in figure 3. On the other hand, the reduced intensity of the D-component upon annealing indicates the so-called 'healing effect', where most of the intrinsic defects in graphene may be removed by the enhanced thermal migration of ions upon annealing at Ta = 300 °C [50].
4. Conclusions
We have studied the modification of the electronic properties of SLG by doping it with slow Li+ ions of energy 5 eV and measuring the changes in the π-band and Li 1s core level with synchrotron photons. Unlike the adsorption of neutral Li atoms on SLG, where bilayer graphene is formed by the intercalated Li atoms below the buffer layer, we find that the Li+ ions doped on SLG at 90 K are not intercalated but cause a significant charge asymmetry between the two carbon sublattices to open a band gap at the K-point of the Brillouin zone. We further observe that the size of the band gap can be artificially adjusted by controlling the ion coverage, θ, followed by proper thermal annealing. The band gap Eg = 0.85 eV we thus obtained upon annealing at Ta = 300 °C from the Li+-doped SLG with θ = 1.0 ML turns out to be the largest band gap reported so far from all functionalized SLG samples, using various kinds of atoms or ions. Furthermore, the band gap thus prepared is found to have the lowest in-gap intensity and is also found to preserve the noble nature of the linear π-band, which is quite distinct from other functionalized SLG samples. The whole fabrication process for Li+-doped SLG, however, has to be done in ultrahigh vacuum conditions (as done in this work), since most alkali metal ions—including Li+ ions—are extremely reactive in air. When several practical and technical issues, such as the air stability of the Li+-adlayer on SLG, are resolved, the improved features of the band gap for Li+-doped SLG deserve to be fully utilized in the development of future graphene-based nano-electronic devices.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (NRF-2015R1A5A1009962), and also by the Ministry of Science, ICT and Future Planning (NRF-2013R1A1A2005598).