Abstract
Special electric and magnetic characteristics make Fe3O4 widely applied in the electromagnetic (EM) wave absorption region. However, for pure Fe3O4, it is still a challenge to simultaneously obtain high absorption intensity and broadband absorption at a low thickness, owing to its low dielectric property. As we realized, flake configuration and the porous structure have obviously promote the EM wave absorption property. Because the former can lead to multi-reflection between flakes and the latter is conductive to interface polarization, flaky Fe3O4 with a porous and coarse surface was designed to overcome the deficiency of traditional Fe3O4 particles. The experimental results demonstrate that the flaky configuration is conductive to enhancing the dielectric coefficient and optimizing impedance matching. Moreover, the complex permittivity rises with the aspect ratio of the sheet. Under a suitable dimension, the flaky Fe3O4 could acquire targeted EM wave absorption capacity in the X band (8–12 GHz). In detail, the maximum reflection loss (RL) could reach a strong intensity of −49 dB at 2.05 mm. The effective absorption bandwidth (EAB) with RL below −10 dB is 4.32 (7.52–11.84) GHz, which is almost equivalent to the whole X band (8–12 GHz). Even more exciting, when regulating the thickness between 2.05 and 3.05 mm, the EAB could cover the entire C and X bands (4–12 GHz). This study provides a good reference for the future development of other ferromagnetic materials toward specific microwave bands.
Export citation and abstract BibTeX RIS
1. Introduction
Ferromagnetic media occupy a crucial position in microwave absorption because of their unique magnetic property and optimal impedance matching [1–4]. However, the decline of permeability in the GHz region caused by Snoek's limit harms not only their magnetic attenuation competency, but also good impedance match. The evident drawbacks have impeded its practical exploitation. As we known, Snoek's limit can be written as follows [5]:
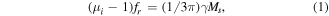
where μi is the initial permeability, fr is the resonance frequency, γ is the gyromagnetic ratio, and Ms is the saturation magnetization. Fortunately, the ferromagnetic flakes may break through the Snoek limitation and offer high permeability values in the GHz range [6]. Combining the Landau–Lifshitz–Gilbert equation with Snoek's law, the following formula can be speculated [7]:
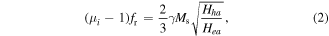
where Hha and Hea stand for the out-plane anisotropy field and in-plane anisotropy field, respectively. With respect to the anisotropy materials, the value of Hha is far greater than that of Hea, and thus, μi and fr should be much bigger than those of isotropy media [8]. As a result, compared with isotropy media, flaky media are much more likely to be a superior absorber because of the large shape anisotropy. Apart from holding the μi value at a high frequency, the flaky absorbers also possess other two attractions. On one hand, the flake framework can restrain the eddy current effect when the thickness is smaller than the skin depth, which would result in less of the microwave being reflected [9]. On the other hand, the individual microstructure is beneficial for improving the dielectric property. Because the flake configuration usually has a large size and surface area, it has better space charge polarization than granular powder [10].
Fe3O4, as a significant member of ferromagnetic materials, has representative magnetism and strong spin polarization grounded to the division of electrons between Fe3+ and Fe2+ in the octahedral sites [11]. For its hypotoxicity, favorable biocompatibility, and low cost, Fe3O4 magnetite has attracted considerable attention in the electromagnetic (EM) wave absorption domain [12]. Nevertheless, single Fe3O4 often suffers from unavoidable intrinsic shortcomings such as inferior dielectric and electric properties, and therefore the desired reflection loss (RL) value in a broad frequency range at a low thickness can hardly be achieved [13, 14]. For overcoming this weakness, integrating Fe3O4 with other typical dielectric dissipation materials, e.g. carbon nanotubes (CNTs), [15] rGO, [16] MnO2, [17] TiO2 [18], has been a popular approach. Although these methodologies have obtained remarkable microwave absorption performances, the relatively complex synthetic procedures, as well as raised costs, inhibit their diffuse applications. Alternatively, it is more advisable to modify the morphology of Fe3O4 to perfect its absorbing capability. As aforementioned, the flaky structure is different for enhanced dielectric capability and attenuating the incident microwave. Therefore, designing flaky Fe3O4 is another valid way to fulfill high-performance EM wave absorbency. For example, Yang et al [19] fabricated Fe3O4 nanodiscs and got a maximum RL value of −37.0 dB.
Recently, porous configurations with a large specific surface area, have been discovered to be useful for accelerating EM wave absorption intensity, owing to their interfacial polarization and multi-reflection [20, 21]. Therefore, the combination of a flaky structure and porous architecture is expected to further improve the EM wave absorbency of Fe3O4. Herein, flaky Fe3O4 with different dimensions are prepared by the reduction of different sizes of α-Fe2O3 flakes using sodium borohydride (NaBH4). These Fe3O4 have an uneven porous surface, and their thicknesses and diameters are about 0.6–0.7 μm and 4.7–6.0 μm, respectively. When the coating thickness is 2.05 mm, the maximum RL value is up to −49.0 dB. The widest effective absorption bandwidth (EAB) of 4.32 (7.52–11.84) GHz at 2.25 mm nearly amounts to whole the X band. Moreover, when the matching thickness is between 2.05 and 3.05 mm, the EAB can even cover the integrated C and X bands.
2. Experimental
2.1. Materials
NaBH4 and sodium hydroxide (NaOH) were bought from Nanjing Chemical Reagent Co. Ferric acetylacetonate (Fe(acac)3) was purchased from the Aladdin Chemistry Co., Ltd. Triethylene glycol (TEG) was bought from Shanghai Titan Scientific Co., Ltd. All ingredients were of analytical grade, and directly used without further purification.
2.2. Synthesis of flaky α-Fe2O3
In typical fashion, the flaky α-Fe2O3 was prepared by the facile solvothermal process. Briefly, 0.6 g of Fe(acac)3 was dispersed in 50 ml of TEG. After stirring for 30 min, a moderate amount of NaOH solution (6 mol L−1) was introduced to the above solution with additional stirring for 1 h. Then, the mixture was transferred into a 100 ml Teflon-lined stainless autoclave, and heated at 200 °C for 4 h. Lastly, the α-Fe2O3 was collected by centrifuge and washed with alcohol and deionized water, respectively, three times. The generated α-Fe2O3 with different aspect ratios were defined as α-Fe2O3-2, α-Fe2O3-10, and α-Fe2O3-20 for the varying NaOH volumes of 2, 10, and 20 ml, respectively.
2.3. Preparation of porous flake-like Fe3O4
First, 0.1 g of α-Fe2O3 and 10 mmol of NaBH4 were added to the 30 ml deionized water, and sonicated for 15 min. Subsequently, the mixture was transferred into a 50 ml Teflon-lined stainless autoclave, and heated at 160 °C for 12 h. The black products were collected by magnetic separation, and washed with ethanol five times. For convenience, the procured Fe3O4 flakes, according to the different sizes of precursor α-Fe2O3, were named as Fe3O4-2, Fe3O4-10, and Fe3O4-20.
2.4. Characterization
The morphologies of all samples were observed by field emission scanning electron microscopy (FESEM, Hitachi S4800). The phase compositions for all samples were identified by an x-ray diffractometer (XRD, Bruker D8 ADVANCE) using Cu Kα radiation (λ = 0.154 178 nm) with an accelerating voltage of 40 kV and applied current of 40 mA. The chemical valence of the Fe element in the sample was measured by x-ray photoelectron spectroscopy (XPS, PHI 5000 Versa Probe). The magnetic characteristics were tested by a vibrating sample magnetometer (VSM, Lakeshore, Model 7400 series). The EM parameters were measured by an Agilent PNA N5244A vector network analyzer based on the coaxial-line method. The specimens were blended with paraffin at a mass proportion of 7:3, and then pressed into toroidal-shaped composites (Φout = 7.0 mm, Φin = 3.04 mm).
3. Results and discussion
The synthetic process for flaky Fe3O4 with a rough porous surface is presented in figure 1. In the first step, by regulating the amount of NaOH, different aspect ratios of α-Fe2O3 flakes were obtained. As shown in figures 2(a)–(c), these α-Fe2O3 samples have an obvious flaky structure with a smooth surface. The diameter and thickness are about 0.14 μm and 80 nm for α-Fe2O3-2, 4.6 μm and 0.6 μm for α-Fe2O3-10, and 5.98 μm and 0.72 μm for α-Fe2O3-20, respectively. Hence, their aspect ratios (the ratio of diameter to thickness) are around 1.75, 7.6, and 8.6 for α-Fe2O3-2, α-Fe2O3-10, and α-Fe2O3-20, respectively. Clearly, with the content of NaOH increasing, the aspect ratios of α-Fe2O3 flakes raises as well. As we know, α-Fe2O3 is a kind of semiconductor with a wide band gap and no magnetism, and thus cannot dissipate microwaves effectively [22]. Innovatively, in the second step, we reduced α-Fe2O3 to Fe3O4 with the help of NaBH4 based on its predominate microstructure. As seen in figure 2(d), none of the flaky configurations are found in the Fe3O4-2 sample, which comprises of many irregular particles and shows some agglomerations. The Fe3O4-10 and Fe3O4-20 inherit the flaky structures of the precursor α-Fe2O3, and the diameters and thicknesses are almost equal to the corresponding α-Fe2O3 (figures 2(e), (f)). Meanwhile, the surface becomes rough and porous in comparison with the precursor α-Fe2O3, which is due to the lost part of the oxygen during the phase transformation process. In terms of equation (3), when 12 mol of α-Fe2O3 are converted to 8 mol of Fe3O4, the 4 mol of O atoms are lost in the system.
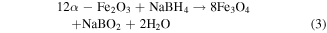
Figure 1. Synthetic route of flake-shaped Fe3O4.
Download figure:
Standard image High-resolution imageFigure 2. FESEM images of (a) Fe2O3-2, (b) Fe2O3-10, (c) Fe2O3-20, (d) Fe3O4-2, (e) Fe3O4-10, and (f) Fe3O4-20.
Download figure:
Standard image High-resolution imageBy further magnifying their FESEM images, it is clear that these pores, with corresponding sizes of ca. 10–110 nm, are randomly dispersed on the flake surface (figure S1 is available online at stacks.iop.org/NANO/29/295603/mmedia). The apparent porous structures can extend the transmission path of the microwave, being advantageous for the microwave attenuation.
The phase information of all samples was determined by XRD analysis. All diffraction signals in figure 3(a) are well matched with the rhombohedral hematite Fe2O3 standard peaks (PDF#33-0664). In figure 3(b), the symbols located at 18.27°, 30.06°, 37.12°, 43.03°, 57.16°, and 62.72° are ascribed to (111), (200), (311), (400), (511), and (440) planes of cubic magnetite Fe3O4 (JCPDS No.: 19-0629), respectively. No other impurity peaks could be observed, demonstrating the α-Fe2O3 were successfully reduced to the Fe3O4. Furthermore, based on Debye–Scherrer's formula [23], the estimated average crystalline size was around 41 nm, 44.3 nm, and 38.1 nm for Fe3O4-2, Fe3O4-10, and Fe3O4-20, respectively.
Figure 3. XRD profiles of (a) Fe2O3 and (b) Fe3O4; (c) Fe 2p XPS spectra of the typical Fe2O3-10 and Fe3O4-10 samples; and (d) M-H loops measured at room temperature.
Download figure:
Standard image High-resolution imageIn order to further identify the chemical valence of iron ions, the XPS profiles are displayed in figure 3(c). The peaks centered at about 709 and 723.1 eV correspond to the Fe 2p3/2 and Fe 2p1/2, respectively [1]. The existence of the satellite peak at around 719.2 eV is characteristic of Fe2O3 [24]. However, no satellite is detected in the curve of Fe3O4, thus ruling out the presence of α-Fe2O3 or γ-Fe2O3 in the Fe3O4 specimens. These conclusions are in accordance with the XRD results, and further prove that thorough phase transformation could be realized by the reduction of NaBH4.
The magnetic properties of specimens were measured by VSM analysis. As displayed in figure 3(d), all three samples present the typical magnetic behaviors. In detail, the saturation magnetization (Ms) values of Fe3O4-2, Fe3O4-10, and Fe3O4-20 are about 85.7, 86.4, and 87.9 emu g−1, respectively, which are quite close to the bulk value of Fe3O4 (92 emu g−1). Meanwhile, the Hc values are 111.4 Oe for Fe3O4-2, 114 Oe for Fe3O4-10, and 116 Oe for Fe3O4-20. The strong magnetism would result in magnetic dissipation, which benefits the EM wave absorption behavior.
The EM wave absorption performances were assessed based on the tested EM parameters. According to the transmission line theory, the RL can be written as follows [25, 26]:
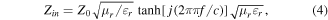
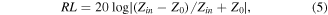
where Zin is the input impedance of the absorber, Z0 represents impedance of free space, f is the frequency, c is the velocity of light, d is the thickness, and εr and μr correspond to the complex permittivity and permeability, respectively. For fulfilling the demand of practical application, the RL value is required to be below −10 dB, indicating that 90% of the incoming EM wave could be absorbed [27]. Figure 4 displays the EM wave absorbency of the three Fe3O4 samples from 1 to 5 mm over the range of 2–18 GHz. For the Fe3O4-2 sample, a maximum RL value of −44.3 dB is obtained at 4.5 mm (figure 4(a)). The large thickness obviously is not suitable for commercial application. Delightedly, the maximum RL value of the Fe3O4-10 sample could reach −49.0 dB at 2.05 mm. Furthermore, the broadest EAB of 4.32 GHz with a frequency from 7.52 to 11.84 GHz almost covers all the X band. Even more interestingly, when adjusting the thickness between 2.05 and 3.05 mm, the EAB could cover nearly all the C and X bands (figure 4(d)). At the same time, it is obvious that the EAB shifts from high frequency to low frequency with increasing thickness. This phenomenon can be interpreted by the quarter-wavelength laws, which illustrate the inverse proportional relationship between matching frequency and matching thickness [20]. Figure 4(c) shows the RL results of Fe3O4-20. Although the RL value of −33.5 dB at 1.9 mm and EAB of 3.88 GHz at 2.15 mm could be achieved, these values are still undesirable compared with those obtained with Fe3O4-10. Ultimately, the Fe3O4-10 sample presents the best EM wave absorption properties among the three Fe3O4 samples, and has great potential to be an outstanding absorber with strong absorbency, wide absorption bandwidth, and low thicknesses.
Figure 4. Two-dimensional RL contour map of (a) Fe3O4-2, (b) Fe3O4-10, and (c) Fe3O4-20. (d) Effective absorption frequency bandwidths between 2.05 mm and 3.05 mm thickness for the Fe3O4-10 sample.
Download figure:
Standard image High-resolution imageFor comprehending the conceivable EM wave absorption mechanism, the EM parameters of three Fe3O4 samples are presented in figure 5. In general, the real parts of complex dielectric (ε') and permeability (μ') relate to the storage competences for electric and magnetic energy; meanwhile, the imaginary parts (ε'' and μ'') represent the attenuation capacities for electric and magnetic energy [28]. From figure 5(a), one can observe that the ε' values are in the ranges of 5.4–6.6, 12.3–13.0, and 13.87–15.2 (with slight fluctuations) for Fe3O4-2, Fe3O4-10, and Fe3O4-20, respectively. The FESEM results show that the Fe3O4-2 sample consists of many anomalous particles, while the other samples show micron-sized flaky architectures. It is obvious that the formation of a flake-shaped structure is conductive to the increase of the dielectric coefficient. Unexpectedly, the Fe3O4-10 and Fe3O4-20 samples have similar flaky configurations, but the ε' values of Fe3O4-20 are higher than those of Fe3O4-10. This can be interpreted by their diverse aspect ratios (Fe3O4-20, 8.6 versus Fe3O4-10, 7.6), which are demonstrated by the FESEM images. The larger aspect ratio and porous characteristics increase the number of dipoles and the area of interfaces, which enhances the dipoles polarization and interfacial polarization at the air–sample interface. Hence, the ε' values rise with the increase in aspect ratio. The phenomenon where ε' rises with increasing aspect ratio in flake-shaped samples has been previously reported [23]. Besides, conductivity may also contribute to ε' values. When the three Fe3O4 samples are dispersed in an insulating paraffin matrix, the larger unique flake structure may be conductive to the formation of partial connectivity, thus leading to an improvement in electrical conductivity, which results in higher ε' values. Additionally, multiple resonance peaks can be distinctly seen in the ε'' curves of Fe3O4-10 and Fe3O4-20, which may be due to the interface polarization caused by the porous structures [29]. Figure 5(b) displays the μ' and μ'' values of the three Fe3O4 samples. The μ' values decline rapidly from 1.65, 1.82, and 1.75 to around 0.8 with increasing frequency for Fe3O4-2, Fe3O4-10, and Fe3O4-20, respectively. Meanwhile, parallel variation tendencies can be found in the μ'' plots, where the values sharply decrease from 0.9, 0.97, and 0.98 to about 0 for Fe3O4-2, Fe3O4-10, and Fe3O4-20, respectively. This phenomenon may result from the relaxation of magnetic moments instead of the magnetic hysteresis; therefore, the testing frequency is higher than the relaxation frequency of the mediums [30]. Furthermore, these original high μ' and μ'' values imply promising impedance matching and strong magnetic loss [31].
Figure 5. Frequency dependence of (a) complex permittivity and (b) complex permeability for all Fe3O4 samples.
Download figure:
Standard image High-resolution imageThe dielectric loss tangent (tan δε = ε''/ε') and magnetic loss tangent (tan δμ = μ''/μ') are calculated to clarify the contribution of different attenuation mechanisms [32]. From figure 6(a), it is observed that the magnetic loss factors of the three Fe3O4 samples are all higher than the dielectric loss during the measured frequency range. Accordingly, the magnetic loss plays a dominant role in dissipating the incident microwave energy. In the microwave region, the magnetic loss mainly derives from the natural resonance, exchange resonance, and eddy current loss [33]. Among them, the eddy current loss may prevent the incoming EM wave from entering into the interior of the absorber for latter attenuation, and should be inhibitive. The eddy current loss coefficient (C0) is connected to the thickness (d) and conductivity (σ) of the absorber, and can be expressed as follows: C0 = μ'' (μ')−2f−1 = 2πμ0σd [34]. According to this formula, if the eddy current loss is the main cause for magnetic loss, the C0 values should remain constant with the variation of frequency. From figure 6(b), it is clear that the C0 values of all specimens decrease as the frequency increases from 4–15 GHz, and then remains constant from 15–18 GHz. Hence, the eddy current loss mainly occurs at high frequency. At low frequency, the magnetic loss competence is mainly aroused from the nature resonance and exchange resonance.
Figure 6. (a) Frequency dependence of dielectric/magnetic loss tangent, and (b) eddy current loss coefficient for the three Fe3O4 samples.
Download figure:
Standard image High-resolution imageBased on the above comparison and discussion, it is concluded that the different microstructures and aspect ratios should be responsible for the distinct dielectric coefficients and EM wave absorption characteristics. It is worth noting that when the complex permeability of samples are at the proximal level, appropriate ε' and ε'' values can meet the demands of optimal impedance matching, and lead to a strong RL intensity and wide absorption bandwidth. Among the three specimens, the Fe3O4-10 medium possesses a moderate aspect ratio and individual micro-architecture, and exhibits prominent EM absorption characteristics. The effect of a filler ratio for Fe3O4-10 in a paraffin matrix on the final EM absorption performance was also investigated. As shown in figure S2, for filler ratios of 60%, the maximum RL value was only −22 dB at 3.8 mm because of the lower permittivity and permeability (figures S2(a), (d)). When the filler content increases to 65%, although the maximum RL peak could reach −56 dB at 3.8 mm (figures S2(b), (e)), the large coating thickness is not desirable. The microwave performance becomes poor upon further increasing the mass percentage to 75% (figures S2(c), (f)), owing to the imbalance between the complex permittivity and complex permeability. The optimal filler proportion was 70%. Under this condition, the maximum RL of −49.0 dB at 2.05 and effective frequency bandwidth of 4.32 GHz at 2.25 mm are achieved. Table 1 lists the EM absorption performances of different morphologies of Fe3O4 samples in previous studies. Compared with these materials, the Fe3O4 sample in this work has a strong RL of −49 dB at a relatively low thickness of 2.05 mm. Furthermore, the EAB of 4.32 GHz can nearly cover the whole X band at 2.25 mm. These outstanding performances suggest that the Fe3O4 sample can be regarded as a candidate microwave absorber with high absorption intensity and broad frequency bandwidth.
Table 1. Comparison of microwave absorption capabilities for different morphologies of Fe3O4 samples.
| Maximum RL | RL ≤ −10 dB | |||||
|---|---|---|---|---|---|---|
| Sample | dm | RLmax | EAB (GHz) | dm | EAB range (GHz) | Reference |
| Fe3O4 nanodisc (20 vol%) | 2.5 | −35.6 | — | — | — | [19] |
| Octahedral Fe3O4 (80 wt%) | 1.4 | −23.6 | 2.3 | 1.4 | 14–16.3 | [35] |
| Hollow Fe3O4 hemispheres (60 wt%) | 3.5 | −31 | 4.2 | 5 | 4–8.2 | [36] |
| Fe3O4 micro-spheres (40 wt%) | 4 | −45.2 | 3.2 | 2.5 | 7.3–10.5 | [37] |
| Dendritic-like Fe3O4 | 5 | −53.0 | 4.5 | 2.0 | 4.0–9.5 | [1] |
| Loose nanoscale Fe3O4 spheres (75 wt%) | 2.5 | −33 | 2.2 | 2.5 | 9.0–11.2 | [38] |
| Flower-like Fe3O4 (50 wt%) | 5 | −30.8 | 2.5 | 3.5 | 7.3–9.8 | [39] |
| Fe3O4 nanoparticles (30 wt%) | 6 | −40.2 | 5.6 | 6 | 2–7.6 | [40] |
| Urchin-like Fe3O4 (50 wt%) | 4 | −29.96 | 2.7 | 3 | 6.3–9 | [41] |
| Flaky porous Fe3O4 (70 wt%) | 2.05 | −49 | 4.32 | 2.25 | 7.52–11.84 | This work |
4. Conclusion
In summary, by adjusting the amount of NaOH, micro-sized α-Fe2O3 flakes with different aspect ratios were fabricated through the solvothermal method. Then, complete phase transformation from α-Fe2O3 to Fe3O4 was fulfilled by a reduction with NaHB4. Apart from the Fe3O4-2 sample, the obtained Fe3O4-10 and Fe3O4-20 samples preserved the flake morphologies, and the surface became rough and porous compared with the corresponding precursor α-Fe2O3. It was found that the dielectric values of Fe3O4 were vitally influenced by their micro-configurations and dimensions. The construction of a flake-shaped structure favored an increasing dielectric coefficient. At the same time, the dielectric values increased with increasing aspect ratios for the flaky framework. The Fe3O4-10 sample exhibited outstanding microwave performance through the strong cooperation between dielectric and magnetic properties. Specifically, the maximum RL of −49.0 dB was obtained at only 2.05 mm. The widest EAB of 4.32 GHz at 2.25 mm could cover almost the whole X band. More interestingly, when the thickness was between 2.05 and 3.05 mm, the frequency range with an RL lower than −10 dB width could spread over both C and X bands.
Acknowledgments
Financial support from the National Nature Science Foundation of China (No. 11575085), the Qing Lan Project, Six Talent Peaks Project in Jiangsu Province (No.XCL-035), Jiangsu 333 Talent Project, the Funding for Outstanding Doctoral Dissertation in NUAA (No. BCXJ17-07), Postgraduate Research & Practice Innovation of Jiangsu Province (KYCX17_0252), and the Priority Academic Program Development of Jiangsu Higher Education Institutions are gratefully acknowledged.







