Abstract
Circulating tumor cells (CTCs), tumor cells that disseminate from primary tumors to the bloodstream, have recently emerged as promising indicators for cancer diagnosis and prognosis. However, the technical difficulties in isolating and detecting rare CTCs have limited the widespread applicability of this method to date. In this work, a new integrated microfluidic system integrating micromixers and micropumps capable of performing 'negative selection and enrichment' of CTCs was developed. By using anti-human CD45 antibodies-coated magnetic beads, leukocytes were effectively removed by applying an external magnetic force, leaving behind an enriched target cell population. The on-chip CTC recovery rate was experimentally found to be 70 ± 5% after a single round of negative selection and enrichment. Meanwhile, CD45 depletion efficiency was 83.99 ± 1.00% and could be improved to 99.84 ± 0.04% after three consecutive rounds of depletion. Notably, on-chip negative selection and enrichment was 58% faster and the repeated depletion could be processed automatically. These promising results suggested the developed microfluidic chip is potentiated for a standardized CTC isolation platform.
Export citation and abstract BibTeX RIS
Nomenclature and abbreviation
| BSA | Bovine serum albumin |
| CNC | Computer-numerical-control |
| CTC | Circulating tumor cell |
| EMT | Epithelial-to-mesenchymal transition |
| EMV | Electromagnetic valves |
| EpCAM | Epithelial cell adhesion molecule |
| ODEP | Optically-induced dielectrophoretic |
| PDMS | Polydimethylsiloxane |
| PMMA | Polymethylmethacrylate |
| RBC | Red blood cell |
| WBC | White blood cell |
Introduction
Circulating tumor cells (CTCs) are tumor cells that disseminate from primary tumors to the bloodstream, and subsequently migrate to distant organs. Therefore, CTCs play a critical role in the initiation of metastasis and the spread of cancer to other places in the body [1], which not only complicates disease management but also causes the death of ~90% of all cancer patients [2]. On the other hand, the occurrence of CTCs in the bloodstream can also function as a 'liquid biopsy' of a tumor that is far less invasive compared to conventional biopsy examination [3]; the detection of CTCs can thus be leveraged for the diagnosis and prognosis of cancer, as well as the real-time monitoring of therapeutic responses. Indeed, clinical studies have demonstrated that the presence of CTCs is correlated with disease progression for a wide range of cancers, such as breast, colorectal, and prostate cancer [4, 5].
Unfortunately, CTCs are extraordinarily rare, with only a few CTCs circulating in the bloodstream along with millions of white blood cells (WBCs) and billions of red blood cells (RBCs), making their isolation a tremendous technical challenge [6]. Thus, the isolation of CTCs from patient blood with high efficiency and high purity has become the focus of intense research efforts. These efforts have resulted in various label-free strategies, which isolate and enrich CTCs based on their inherent difference in size, density, electric charge, and deformability from normal blood cells [7]. However, perhaps the most commonly used method to date still relies on the specific interaction of antibodies and the epithelial cell adhesion molecule (EpCAM)—the most common tumor-associated antigen and frequently overexpressed in solid tumors—to capture and isolate CTCs [8, 9]. For instance, the CellSearch system is a FDA approved technology that employs such an EpCAM antibody-based 'positive selection' method for isolating CTCs and has been utilized in clinical applications [4]. Nevertheless, the main drawback for positive selection is its inability to isolate all types of CTCs, which are known to be heterogeneous [10]. For instance, cancer cells may undergo epithelial-to-mesenchymal transition (EMT) and down-regulate the expression of EpCAM [11]. Moreover, CTCs from EpCAM negative tumors, such as soft-tissue tumors, lymphomas and even some carcinomas may also not be detected by this approach [12].
In order to address the limitations of positive selection, an alternate approach referred to as 'negative selection and enrichment' has been reported [13–16]. In this approach, RBCs in patient blood samples are first removed by either lysis or gradient separation, and leukocytes are subsequently immune-depleted from the sample by leveraging common leukocyte-specific antigen CD45. Importantly, because CD45 is not expressed on cancer cells, ideally, all types of CTCs, either epithelial marker positive or negative, remain in the sample, facilitating their enrichment and recovery. Furthermore, by obviating antibody-based capture and isolation, innate characteristics of enriched CTCs can be better determined. Negative selection and enrichment of CTCs may therefore represent an unbiased approach for CTC isolation [17].
In this study, a new integrated microfluidic chip capable of automating negative selection and enrichment of CTCs was developed. Critical steps of the enrichment process, including sample mixing, magnetic capture, and sample washing can be precisely performed in the chip without human intervention. Here, using a human colon carcinoma cell line HCT8 as the target rare cells (i.e. model CTCs) and immortalized human T lymphocytes Jurkat as a non-target, CD45+ background cells, the device depleted 83.99 ± 1.00% of CD45+ cells and recovered 70 ± 5% of model CTCs in ~17 min, a 58% reduction in time when compared to the conventional method (~40 min). Importantly, the microfluidic chip approach also enabled efficient execution of multiple rounds of negative selection, improving the depletion to 99.84 ± 0.04% of the total CD45+ cells after triple depletion in under 50 min. These promising results suggested the developed chip may be useful for standardized CTC isolation platforms.
Materials and methods
Chip design and fabrication
A schematic illustration and a photograph of the CTC negative selection and enrichment chip are shown in figure 1(a). Dimensions of the microfluidic chip were measured to be 45 × 25 mm (length × width). The chip integrates two different sizes of circular membrane micropumps, including a larger micromixer (diameter: 5 mm, fluidic chamber depth: 150 μm, membrane thickness: 150 μm) and a smaller micropump (diameter: 2 mm, fluidic chamber depth: 150 μm, membrane thickness: 150 μm), and several normally-closed microvalves to control liquid transport and mixing. In addition, the chip contains three reservoirs for storing cell mixture, magnetic beads, and wash buffer, respectively. Electromagnetic valve (EMV)-controlled pressure and vacuum sources were connected to the device via eight air holes to control the actuation of the micromixer, the micropump, and the microvalves. The waste reservoir was connected to a separate vacuum source to collect the waste throughout the process of CTC negative selection and enrichment. An external magnet was placed beneath the micromixer chamber to trap cell-bead complexes, which are then removed during the washing step with the external magnet also removed.
Figure 1. Microfluidic CTC negative selection and enrichment device. (a) An illustration and a photograph. The red color and the blue color in the photograph designate the liquid transport channels and the air control channels, respectively; (b) a side view of the micromixer membrane actuation process at the end of mixing.
Download figure:
Standard image High-resolution imageAn exploded view of the chip is illustrated in figure S1 (stacks.iop.org/JMM/25/084007/mmedia). The chip was composed of an air control layer (upper layer), a liquid transport layer (bottom layer) and a glass substrate. In brief, master molds of microstructures on polymethylmethacrylate (PMMA) plates were first formed by using a computer-numerical-control (CNC) machine (EGX-400, Rolan Inc., Japan) equipped with a 0.5 mm drill bit. Then a polydimethylsiloxane (PDMS) replica-molding process was performed to fabricate the air control layer and the fluidic channel layer. The PDMS layers were first bonded to each other following oxygen plasma treatment (Plasma Cleaning Model CUTE MP-R, UVOTECH Systems, USA), and the bonded PDMS layers were finally bonded to the glass substrate following another oxygen plasma treatment to form the complete microfluidic chip.
In order to pneumatically drive the micromixer, the micropump, and the microvalves on the chip, a pressure source (~25 kPa) and a vacuum source (~90 kPa) were connected to a set of EMVs (S070M-5BG-32, SMC Inc., Japan), which were then connected to the air control holes on the chip via the tubing. Pressure was applied at the end of the mixing sequence to push the membrane down to the bottom glass substrate and squeeze any remaining liquid out of the micromixer chamber to minimize the sample liquid loss in the microfluidic chip (figure 1(b)). Actuation sequences of the micro-components to pump, mix, and transport liquid samples were achieved by controlling the opening and closing of each EMV with a programmable control circuit [18]. The programmble actuation of these micro-components enabled automated liquid handling, thereby obviating manual operation during negative selection and enrichment of CTC.
Experimental procedure
Figure 2 illustrates the experimental procedure implemented on the CTC negative selection and enrichment chip. Prior to the experiment, a cell mixture containing lymphocytes and cancer cells, anti-human CD45 antibodies-coated magnetic beads, and a wash buffer (Ca2+ and Mg2+ free PBS, pH 7.4) were loaded into their designated reservoirs. To initiate the experiment, 20 μl of magnetic beads were transported by the micropump to the cell reservoir (figure 2(a)). Cells and beads were mixed gently in the micromixer chamber for 10 min to facilitate cell-bead binding (figure 2(b)). Subsequently, an external permanent magnet was placed underneath the micromixer to capture the cell-bead complexes (figure 2(c)), while unbound cells remaining in the cell suspension were transported back to the cell reservoir (figure 2(d)). Because a significant portion of background cells were depleted from the original cell suspension, target cancer cells in the suspension became enriched. Before starting another round of depletion, the magnet was removed and the cell-bead complexes in the micromixer chamber were washed away by continuously pumping 1 ml of the wash buffer through the chamber (figure 2(e)). Finally, multiple rounds of lymphocyte depletion could be accomplished by repeating the steps 2(a) through 2(e), which were performed in an automated fashion.
Figure 2. Experimental protocol performed on the integrated CTC selection and enrichment chip. (a) Addition of anti-CD45 magnetic beads; (b) mixing and incubation; (c) collection of cell-bead complexes by using an external magnet; (d) transportation of enriched suspension for next depletion or analysis after multiple detection; (e) removal of cell-bead complexes and readying the micromixer for the next depletion.
Download figure:
Standard image High-resolution imageSample preparation
Colorectal cancer is one of the most common cancers in the world, and thus we chose human colon carcinoma cell line HCT8 as the target rare cells (i.e. model CTCs). Immortalized human T lymphocytes Jurkat served as non-target background cells that would be depleted during the negative enrichment process. HCT8 and Jurkat cells were both cultured in RPMI 1640 medium (Life Technologies, USA) containing 10% fetal bovine serum (Life Technologies) and incubated at 37 °C with 5% CO2 in air. In this work, Dynabeads® CD45 (Life Technologies) were used to capture and deplete lymphocytes (i.e. Jurkat cells). Per manufacturer's instructions, an isolation buffer (Ca2+ and Mg2+ free PBS supplemented with 0.1% bovine serum albumin (BSA) and 2 mM EDTA, pH 7.4) was used throughout this work for the binding between CD45+ cells and anti-human CD45 antibody-coated magnetic beads. The bead concentration was adjusted to 3.5 × 108 per ml prior to use.
To quantify the number of rare cells in our model sample, HCT8 cells were stained with 0.1 M CellTraceTM Calcein Green AM (Life Technologies), counted using a hemacytometer, and re-suspended in the isolation buffer at 104 cells ml−1. The number of HCT8 in a constant volume (10 μl) was again counted under a fluorescent microscope (Bx43, Olympus, Japan) to verify that the cell suspension contained ~100 cells before the cell suspension was added to 106 Jurkat cells. The volume of the final cell suspension containing both types of cells was 120 μl.
Evaluation of microfluidic chip
To evaluate the volume loss in the micromixer, 120 μl of water was first weighed and loaded into the cell reservoir. After actuating the micromixer at a pulsation frequency of 0.5 Hz for 5 min, the water sample was collected from the cell reservoir and weighed again. The weights before and after were used to determine the volume loss. The viability of cells after they were subjected to microfluidic mixing at various pumping frequencies was also assessed. In each experiment, 106 HCT8 cells were mixed in the micromixer operating at a particular pumping frequency such as 0 Hz, 0.05 Hz, and 0.5 Hz for 15 min. The cells were then retrieved from the chip and examined by Trypan blue staining.
Depletion efficiency of CD45+ cells and recovery of CTCs
The depletion efficiency of the CD45+ cells on the benchtop and in the microfluidic chip were both investigated in the study. The conventional CD45 depletion on the benchtop was performed in accordance with the manufacturer's instructions. In brief, 106 Jurkat cells and 7 × 106 magnetic beads were incubated in a 1 mL isolation buffer for 30 min at 4° C with gentle tilting and rotation. Using a benchtop magnetic particle separator (MPC, Dynabeads® MPC®-1, Life Technologies), the supernatant was collected and the unbound cells remaining in the supernatant were counted by an auto cell counter (TC10, Bio-Rad, USA). On the other hand, on-chip CD45 depletion was performed using the same amount of cells and magnetic beads, but the total reaction volume was decreased to 140 μl. Cells and beads were incubated by actuating the micromixer at a pulsation frequency of 0.5 Hz for 10 min under room temperature. Using an external permanent magnet, the cell-bead complexes were captured and the unbound cells remaining in the cell suspension were transported back to the cell reservoir.
The depletion efficiency of the CD45+ cells was calculated as follows:

CTCs recovered from the supernatant after negative selection and enrichment were observed by fluorescent microscope. The recovery rate of CTC was calculated as follows:
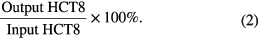
Notably, the large numbers of input and output Jurkat cells that were counted by the auto cell counter afforded a precise calculation of the depletion efficiency (i.e. many significant figures). On the other hand, the small number of input and output HCT8 cells, which were counted manually via fluorescence microscopy, resulted in a recovery rate with only a few significant figures.
Results
Optimization of experimental conditions for negative selection and enrichment
Because the number of CTCs is low, it is imperative for the microfluidic chip to have negligible loss of any sample liquid that may contain CTCs during the enrichment process. The performance in liquid loss was verified by measuring the weight of the liquid samples before loading into the chip and after micromixing and recovering from the chip. Figure 3(a) shows that the liquid weight recovered from the chip was similar to the input weight, suggesting that, under the appropriate membrane actuation, the microfluidic chip has negligible liquid loss.
Figure 3. Characterization of CTC negative selection and enrichment chip. (a) Net weight recovered from the micromixer after mixing at a pulsation frequency of 0.5 Hz for 5 min was similar to that without mixing. (b) Cells remained viable under a pulsation frequency of 0.05 Hz for 15 min. (NS: no siginificant difference)
Download figure:
Standard image High-resolution imageTo avoid potential cell damage and even death by shear forces generated from membrane actuation in the mixing step, different pulsation frequencies of the membrane micromixer were tested. To do so, 106 HCT8 cells were first subjected to micromixer actuation at a particular frequency for 15 min. The cells were then retrieved from the chip and examined after Trypan blue staining. As shown in figure 3(b), the cell viability was 89.5 ± 0.7% at a pulsation frequency of 0 Hz (i.e. no membrane actuation), which was similar to the cells placed on the benchtop for the same time (90.5 ± 0.7%). This suggests that cells stay alive while entering the device and in contact with PDMS. Meanwhile, a comparable 90.0 ± 1.4% of cells remained viable under a pulsation frequency of 0.05 Hz. However, the cell viability dramatically decreased to 58.0 ± 1.4% when the micromixer was operated at a 0.5 Hz pulsation frequency. These results suggest that actuating the membrane micromixer at 0.05 Hz, which still achieved effective mixing (figure S2 (stacks.iop.org/JMM/25/084007/mmedia)), was gentle enough to maintain cell viability.
In order to maximize the success of negative selection and the enrichment of CTCs, an efficient depletion of CD45+ cells must be achieved. As a parameter to optimize the depletion of CD45+ cells, the optimal ratio between magnetic beads coated with anti-human CD45 antibodies and CD45+ cells was measured. Here, a cell suspension containing 106 of Jurkat cells and the antibody-coated magnetic beads were loaded into their designated reservoirs in advance. The magnetic beads were transported to the cell reservoir and mixed with cells by activating the micromixer at 0.05 Hz for 15 min. After mixing and incubation, an external permanent magnet was placed underneath the micromixer to capture the cell-bead complexes, while the cells remaining in the suspension were transported back into the cell reservoir and recovered. The cell-bead complexes in the micromixer were washed away. The recovered cells in suspension were counted and the cell depletion efficiency was calculated using equation (1). The results for the different bead-to-cell ratios under this single round cell depletion are shown in figure 4(a). At a low, 2:1 bead-to-cell ratio, only 40.71 ± 6.18% of the original CD45+ cell population was depleted. Increasing the bead-to-cell ratio improved the depletion efficiency. For example, when the bead-to-cell ratio was increased to 6:1, a significantly higher 75.13 ± 4.42% of CD45+ cells was depleted from the original cell suspension. Further increasing the ratio to 7:1 resulted in a small improvement in the depletion efficiency (79.70 ± 2.07%) without significant difference to the ratio of 6:1, suggesting that the depletion efficiency had likely plateaued. This 7:1 ratio was therefore used in all subsequent experiments.
Figure 4. Parameter optimization for on-chip CD45 depletion. (a) Beads and cells were mixed under a pulsation frequency of 0.05 Hz for 15 min. The depletion efficiency of the CD45+ cells plateaued at 79.70 ± 2.07% at a bead-to-cell ratio of 7:1. (b) On-chip CD45 depletion efficiency for 10 min was comparable to that of using the conventional benchtop method for 30 min. (NS: no siginificant difference)
Download figure:
Standard image High-resolution imageIn comparison to depleting the CD45+ cells in the conventional benchtop set-up, the efficient micromixing of cells and antibody-coated beads in the microfluidic chip facilitated more efficient binding and hence shortened the required incubation time for a single round of cell depletion. For instance, as shown in figure 4(b), 80.88 ± 1.35% of the CD45+ cells were depleted after incubating the sample with a benchtop rotator for an optimal incubation time of 30 min. In contrast, on-chip mixing for only 10 and 15 min led to comparable depletion efficiencies of 78.81 ± 6.40% and 81.08 ± 1.60%, respectively. Of note, the depletion efficiency dramatically decreased to 26.49 ± 0.35% when mixing for 8 min, suggesting an insufficient incubation time. On-chip CD45 depletion efficiency at room temperature or 4 °C was not significantly different (data not shown). Based on these results, the employment of the microfluidic chip at room temperature could shorten the required time for a single round of cell depletion from 30 min to 10 min, or a 67% decrease. The complete on-chip negative selection and enrichment process could be done in ~17 min, while the conventional method needed ~40 min, reducing the time required by 58% and avoiding labor-intensive work.
Multiple leukocyte depletion efficiency and CTC recovery rate
Similar to a previous demonstration in a benchtop set-up, which showed that the depletion efficiency of CD45+ cells in the negative enrichment approach could be increased by performing multiple rounds of cell depletion [15], the microfluidic chip in the current work was also capable of improving the depletion efficiency via multi-round depletion. In microfluidic on-chip multi-round depletion, after transporting the cell suspension that contains unbound CD45+ cells to the cell reservoir and washing cell-bead complexes from the micromixer chamber, a new round of depletion was initiated by transporting fresh beads to the cell reservoir and mixing with CD45+ cells remaining in the cell suspension. The results for multi-round depletion are shown in figure 5. Compared to single depletion, which achieved a depletion efficiency of 83.99 ± 1.00%, two rounds of depletion yielded a significantly improved depletion efficiency of 98.15 ± 0.28%. Three rounds of depletion further enhanced the performance of 99.84 ± 0.04%. However, the depletion efficiency appeared to have plateaued after three rounds of depletion, as four rounds and five rounds of depletion yielded 99.68 ± 0.05% and 99.64 ± 0.11%, respectively, which were comparable to that from three rounds of depletion. Importantly, performing multiple rounds of cell depletion in microfluidic chips was precise and automated. Carrying out this repetitious operation in microfluidic chips presents a significant advantage over a similar operation in a benchtop set-up, which often involves manual steps, and could therefore become cumbersome and error-prone.
Figure 5. On-chip CD45 depletion efficiency after multiple rounds of depletion. The depletion efficiency of CD45+ cell reaches a plateau at 99.84 ± 0.04% after three rounds of depletion.
Download figure:
Standard image High-resolution imageTo evaluate the CTC recovery rate after multi-round depletion, one hundred fluorescently-stained HCT8 cells were spiked into a 106 CD45+ cell suspension. Multiple rounds of negative selection and enrichment were performed, the recovered CTCs in suspension were counted, and the recovery rate was calculated using equation (2). The results for multi-round cell depletion are shown in figure 6. After a single round of CD45 depletion, 70 ± 5% of HCT8 cells remained in the cell suspension. The recovery rate decreased to 53 ± 4% after repeated depletion, while after three rounds of depletion, 32 ± 3% of the spiked HCT8 cells were recovered. Taking the CD45 depletion results and the HCT8 cell recovery results together, the study suggested that the chip could indeed enrich HCT8 cells—and hence CTCs in general—by depleting CD45+ cells. Indeed, a single round of negative enrichment resulted in 70% recovery of 100 HCT8 cells and 83.99% depletion of 106 CD45+ cells. Of note, although the enrichment of HCT8 cells after two rounds of negative selection (53% recovery of 100 HTC8 cells and 98.15% depletion of 106 CD45+ cells) compared less favorably to three rounds of negative selection (32% recovery of 100 HTC8 cells and 99.84% depletion of 106 CD45+ cells), the higher recovery could still render this condition more favorable, especially when downstream analyses demand a higher number of target cells.
Figure 6. On-chip CTC recovery rate. On-chip CTC recovery rate decreases from 70 ± 5% to 32 ± 3% after multiple rounds of depletion.
Download figure:
Standard image High-resolution imageDiscussion
The advantages of negative enrichment in isolating heterogeneous CTCs that would be otherwise neglected by the positive selection approach have recently propelled its development. For example, a CTC-iChip was developed to isolate CTC by either positive (EpCAM) or negative (CD45 and CD15) enrichment [19]. The depletion of nucleated cells from CTC-iChip by negative enrichment was 2.5 log, which was similar to our results after three rounds of depletion (equivalent to 2.8 log based on our calculation). Furthermore, Kyun et al developed a geometrically activated surface interaction (GASI) chip with immobilized CD45 antibodies inside the channel [20]. The enrichment fold of the GASI chip was ~130, and ~50% of 100 spiked cells were recovered. In our system, the enrichment fold was ~200 after three rounds of depletion, which outperformed the GASI chip, but our recovery rate was worse. Overall, our device provides an alternative approach to achieve negative enrichment in microfluidic chips and delivers a comparable performance to existing chip-based negative enrichment methods.
Despite the advantage in unbiased isolation of CTCs, the yield and purity reported by negative enrichment methods have yet to match their positive selection counterparts. For example, our device yielded a relatively low recovery rate of target cancer cells (32%) after multiple rounds of depletion. In order to improve these, both the depletion efficiency of CD45+ cells and the recovery rate of target cancer cells in the microfluidic chip should be improved. Specifically, althugh more than 99% of Jurkat cells were highly expressed with CD45 (data not shown), the depletion efficiency was only ~80% for a single round of depletion and cell-bead complexes could be observed in the suspension after depletion, suggesting that insufficient magnetic capture forces could be the cause of the relatively low depletion efficiency. Thus, magnetic field concentrators [21] could be implemented in future iterations of the device to enhance the magnetic capture forces. Likewise, improving the relatively low CTC recovery, which likely stemmed from cells lodging in the device, via device surface modification or further optimizing the assay parameters (e.g. incubation volume and washing duration) is also a topic for our future investigation.
Recently, negative enrichment was mostly used to combine with positive selection or other selection according to physical difference to achieve the final CTC isolation [22, 23]. Our previous work has developed an optically-induced-dielectrophoretic (ODEP) force-based microfluidic platform to further purify cancer cells from samples processed by conventional, benchtop-based negative selection and enrichment process [24]. By using ODEP force-based cell manipulation, most of the leukocytes were excluded from the mixed leukocyte and cancer cell suspension. The methodology was proven to increase the cancer cell isolation purity to more than 64%, and has great potential to integrate with the present chip to achieve better CTC isolation purity. The developed chip in this study was able to accelerate the process of negative selection and enrichment of CTCs with the added benefit of automation.
Conclusions
In summary, we demonstrated an integrated microfluidic chip capable of performing 'unbiased' CTC negative selection and enrichment with comparable performance as a conventional benchtop method while offering significant advantages in rapid incubation and potential for full automation. By using magnetic beads coated with antibodies against human CD45 antigen, leukocytes were removed by external magnetic force, leaving behind an enriched target cell population. A single round of negative enrichment resulted in 70 ± 5% recovery of 100 HCT8 cells and 83.99 ± 1.00% depletion of 106 CD45+ cells within ~17 min, a 58% reduction in time. The depletion could be improved to 99.84 ± 0.04% by three rounds of depletion, while the recovery of 100 HCT8 was decreased to 32 ± 3%. Higher recovery could render a single round of depletion more favorable because CTCs are rare in blood. In the present study, we focused on demonstrating the feasibility of CTC negative selection and enrichment in microfluidic chips. Therefore, we used human T lymphocyte cell line Jurkat instead of human whole blood to simplify the enrichment process. With further improvements and integration with other microfluidic modules, this chip may present a useful tool for the isolation of rare CTCs from blood, thereby facilitating improved diagnosis, prognosis, and treatment of cancer.
Acknowledgments
The authors would like to thank the Ministry of Science and Technology and Hsinchu Science Park, Taiwan for their financial support (NSC 102-2221-E-007-054-MY3; 103A03). Partial financial support from the 'Towards A World-class University Project' and National Health Research Institutes, the 'Innovative Research Grant' (IRG, NHRI-EX104-10428EI) is also greatly appreciated. Kuangwen Hsieh was supported by a scholarship from the Whitaker International Program administered by the Institute of International Education.
Footnotes
- 6
Preliminary results of the current paper were presented at Micro TAS 2014, San Antonio, Texas, USA, October 26–30, 2014.







