Abstract
In this paper, we show a fabrication method and the dielectric properties of strontium titanium oxynitride (SrTiO3:N) single crystals. Oxynitride single crystals were prepared by annealing SrTiO3 single crystals in gaseous ammonia. SrTiO3:N was assumed to have the chemical composition SrTiO3−3xN2x, which contained oxygen vacancies. To reduce the number of oxygen vacancies, SrTiO3 crystals co-doped with nitrogen and niobium (SrTiO3:N,Nb) were fabricated. The semiconducting Nb-doped SrTiO3 crystals changed to dielectric N,Nb-codoped SrTiO3 crystals with a resistivity of 6 × 1012 Ω·cm with annealing in gaseous ammonia. XPS measurement indicated that niobium doping was effective for increasing the amount of dopant nitrogen. The dielectric permittivity increased with the amount of dopant nitrogen, indicating the effectivity of nitrogen doping for increasing the dielectric permittivity of perovskite oxides.
Export citation and abstract BibTeX RIS
1. Introduction
Perovskite-type oxides (ABO3) are the most widely used ferroelectric materials and their excellent dielectric properties have attracted much attention not only in the electroceramic industry but also in fundamental research.1–10) The polarization mechanism of dielectric properties and the origin of ferroelectricity are closely related to the covalency of A–O and B–O.11–18) Therefore, the dielectric and ferroelectric properties of perovskite-type oxides change depending on the A- and B-site cations. Various combinations of A- and B-site cations in ABO3 have been studied to improve dielectric, ferroelectric, and piezoelectric properties, even seventy years after the discovery of ferroelectric perovskite-type oxides. On the other hand, the anion substitution of the O-site is also attractive for controlling the covalency between cations and anions. Recently, several researchers have reported the anion substitution of dielectric perovskite-type oxides, e.g., Ba1−xKxTiO3−xFx,19–22) (BaxSr1−x)1+yTi1−yO3−zNz,23) and SrTaO2N.24–29) However, there have been few research studies owing to the difficulty in the synthesis of such oxides, and thus the substitutional effect of anions on the dielectric properties remains unknown.
The study presented herein was focused on strontium titanium oxynitride (SrTiO3:N), which is SrTiO3 substitutionally doped with nitrogen. Nitrogen-doped SrTiO3 has been recently attracting attention as a photocatalyst that responds to visible light. Thus, the optical properties and photocatalytic activity of SrTiO3:N particles and thin films have been reported by many researchers.30–33) However, the dielectric properties of SrTiO3:N have remained unknown. Since nitrogen has a smaller electronegativity than oxygen, the covalency between Ti and O/N in SrTiO3:N should be higher than that between Ti and O in pure SrTiO3. The high covalency weakens the short-range repulsion forces against long-range dipolar interaction.11–13,19–22) Therefore, substitution with nitrogen may enhance the ionic polarizability of SrTiO3.
In this paper, we show a fabrication method and the dielectric properties of strontium titanium oxynitride single crystals. Strontium titanium oxynitride single crystals were prepared by annealing SrTiO3 and Nb-doped SrTiO3 single crystals in gaseous ammonia, and the substitutional effect of nitrogen on their dielectric properties was discussed.
2. Experimental procedure
As raw materials for oxynitride single crystals, (100)-oriented SrTiO3 and 0.1 at. % Nb-doped SrTiO3 (SrTiO3:Nb) single crystals purchased from Shinkosha were used. The sample was 10 × 10 × 0.5 mm3, and both of its surfaces were polished to a mirror finish. The single crystals were annealed in gaseous ammonia (NH3) at 1000 °C using a tubular electric furnace. The flow rate of NH3 gas was 0.1 L/min, and the annealing time was varied from 1 to 24 h.
The crystal structures of the nitrided samples were evaluated by powder X-ray diffraction (XRD) with Cu Kα rays (Rigaku RINT-2000) after milling the samples. Microstructures were observed by scanning electron microscopy (SEM; JEOL JCM-6000 NeoScope). UV–vis transmittance spectra of the samples were recorded on a UV–vis spectrophotometer (JASCO V-570). The dopant nitrogen ions in the samples were semi-quantitatively measured by X-ray photoelectron spectroscopy (XPS) with Al Kα ray (ULVAC-PHI PHI5000 VersaProbe II). The diameter of the irradiated area was 100 µm and the constant pass energy was 58.7 eV. The photoelectrons of the N 1s orbital were recorded with a takeoff angle of 45°. The dielectric properties were measured using an impedance analyzer (Agilent 4294A) in the frequency range from 1 kHz to 10 MHz after depositing Au electrodes on the top and bottom surfaces by DC sputtering. Resistivity was measured using a digital ultrahigh resistance meter (ADC 8340A).
3. Results and discussion
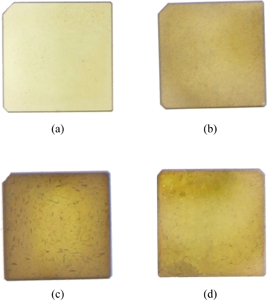
Figure 1 shows photographs of the SrTiO3 single crystals annealed in NH3 gas for various annealing times. The SrTiO3 crystals annealed in NH3 gas changed from colorless to yellow, and the yellow color became deeper with increasing annealing time. XRD measurement indicated that the obtained samples had a perovskite structure without impurities, as shown in Fig. 2. The positions of diffraction peaks seemed to be nearly unchanged before and after annealing treatment, suggesting that the lattice parameters were hardly changed by the nitridation. In addition to the ionic radius of N3− (1.46 Å) being close to that of O2− (1.38 Å), the amount of nitrogen substitution might not be large enough to change the lattice parameters. Figure 3(a) shows the UV–vis transmittance spectra of the samples. The absorption edge of the transmittance shifted to the long wavelength side with increasing annealing time. Miyauchi et al. have reported the electronic densities of states (DOSs) of SrTiO3−2xNx and Sr1−xLaxTiO3−xNx calculated by first-principles calculation.30) According to their calculation results, the energy level of N 2p orbitals in SrTiO3−2xNx and Sr1−xLaxTiO3−xNx was above that of O 2p orbitals, and the mixed orbitals of N 2p and O 2p compose the valence band of SrTiO3−2xNx and Sr1−xLaxTiO3−xNx, whereas the valence band for pure SrTiO3 consists of O 2p orbitals. Therefore, the band gap of N-doped SrTiO3 should be narrower than that of pure SrTiO3. The shift of the absorption edge in our study corresponded to the narrowing of the band gap, suggesting that the nitrogen substitution of oxygen sites in SrTiO3 progressed with the annealing in NH3 gas flow.
Fig. 1. Photographs of SrTiO3 single crystals annealed in NH3 gas for (a) 1, (b) 6, (c) 12, and (d) 24 h.
Download figure:
Standard image High-resolution imageFig. 2. XRD profiles of SrTiO3 single crystals annealed in NH3 gas for various annealing times.
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageFig. 3. UV–vis transmittance spectra of single crystals annealed in NH3 gas: (a) SrTiO3 and (b) SrTiO3:Nb.
Download figure:
Standard image High-resolution imageIf the nitrogen is substitutionally doped into oxygen sites without forming oxygen vacancies, N-doped SrTiO3 should display p-type conductivity to maintain the charge balance in the crystal. However, it is generally very difficult to obtain p-type conductivity in an oxide semiconductor with a wide band gap because the upper level of the valence band is deep and the acceptor level is thermodynamically unstable. Therefore, most of N-doped oxides have oxygen vacancies to maintain charge balance in the crystal.30) From this viewpoint, it is reasonable to think that N-doped SrTiO3 has the chemical composition SrTiO3−3xN2x, which has oxygen vacancies. Figure 4 shows SEM images of the SrTiO3 crystals annealed in NH3 gas for 24 h. Many microcracks along the 〈100〉 direction were observed in the samples annealed for more than 12 h. The samples annealed for more than 24 h were so brittle that they can be broken by hand. Shkabko et al. have reported that stacking faults originated from the oxygen vacancies along the 〈100〉 direction in SrTiO3−3xN2x thin films.34) In addition, oxygen vacancies may cause the reduction in dielectric permittivity. Therefore, it is necessary to reduce the number of oxygen vacancies to prevent reductions in mechanical strength and dielectric permittivity.
Download figure:
Standard image High-resolution imageFig. 4. SEM images of SrTiO3 crystals annealed in NH3 gas for 24 h: (a) surface and (b) cross section.
Download figure:
Standard image High-resolution imageTo reduce the number of oxygen vacancies by nitrogen doping, the doping with high-valence cations may be effective. In this study, a Nb-doped SrTiO3 crystal was annealed in NH3 gas for 12 h and thus a SrTiO3 crystal co-doped with nitrogen and niobium (SrTiO3:N,Nb) was obtained. The chemical formula of the obtained SrTiO3:N,Nb was expected to be SrTi1−2yNb2yO3−3x+yN2x, indicating that the reduction in the number of oxygen vacancies is proportional to that in the amount of doped Nb. By annealing the Nb-doped SrTiO3 crystal in NH3 gas, the crystal was changed from dark blue to yellow. The transmittance spectra shown in Fig. 3(b) indicate a shift of the absorption edge to higher wavelengths, corresponding to the narrowing of the band gap. In addition, the red absorption at approximately 800 nm decreased with SrTiO3:Nb nitridation, suggesting the oxidation of Ti ions. The resistivity of the SrTiO3:N,Nb crystal was 6 × 1012 Ω·cm, whereas that of the pre-annealed SrTiO3:Nb crystal was 8 × 10−2 Ω·cm. This result indicates that the SrTiO3:Nb crystal changed from a semiconductor to an insulator with the nitridation. Figure 5 shows XPS N 1s signals of the SrTiO3:N and SrTiO3:N,Nb crystals annealed in NH3 gas for 12 h. A larger peak at 395 eV and a smaller peak at 398 eV were detected for the SrTiO3:N and SrTiO3:N,Nb crystals, while no peak of nitrogen was detected for the pre-annealed SrTiO3 and SrTiO3:Nb crystals. The N 1s peak at 398 eV was attributed to nitrogen being substituted for oxygen in the perovskite structure, and the peak at 395 eV to the N3− state of the Ti–N bond.23) Therefore, nitrogen ions certainly substituted for oxygen ions and bound covalently to Ti ions. The N 1s peaks of SrTiO3:N,Nb crystal were higher than those of the SrTiO3:N crystal, indicating that the doping with niobium was effective for increasing the amount of doped nitrogen.
Fig. 5. XPS N 1s signals for SrTiO3:N and SrTiO3:N,Nb crystals. Both were annealed in NH3 gas for 12 h.
Download figure:
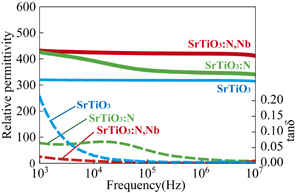
Standard image High-resolution imageFigure 6 shows the dielectric permittivities and loss tangents of the SrTiO3, SrTiO3:N, and SrTiO3:N,Nb crystals. The relative permittivities of the SrTiO3:N and SrTiO3:N,Nb crystals were respectively 340 and 410 at 1 MHz, which were higher than that of the pure SrTiO3 crystal (310). In particular, for the SrTiO3:N,Nb crystal, specimens of two thicknesses (0.46 and 0.25 mm) were prepared by polishing, and it was confirmed that the difference in permittivity between the two specimens was less than 2%. These results suggested that the composition was nearly uniform in the thickness direction, and that the permittivity increased with increasing amount of doped nitrogen. Furthermore, the loss tangent at a low frequency decreased with the nitrogen doping because the substitution by nitrogen was effective for decreasing the donor concentration in the crystal. These results showed the effectivity of nitrogen doping for enhancing the dielectric permittivity. As mentioned in Sect. 1, the covalency between Ti and O/N in SrTiO3:N should be higher than that between Ti and O in pure SrTiO3, weakening the short-range repulsion forces against long-range dipolar interaction. Therefore, the ionic polarization, which is the dominant polarization mechanism of dielectric permittivity for pure SrTiO3,7) might increase with nitrogen substitution. In contrast, oxygen vacancies in SrTiO3:N might suppress dielectric permittivity.
Fig. 6. Dielectric permittivities and loss tangents of SrTiO3, SrTiO3:N, and SrTiO3:N,Nb crystals. The SrTiO3:N and SrTiO3:N,Nb crystals were prepared by annealing SrTiO3 and SrTiO3:Nb crystals in NH3 gas for 12 h.
Download figure:
Standard image High-resolution imageIn further study, the short-range repulsion forces of SrTiO3:N should be simulated by first-principles calculation, and the ionic polarization of SrTiO3:N should be quantitatively evaluated. Far-infrared ellipsometry and THz time-domain spectroscopy are powerful tools for measuring dielectric properties in the THz region for estimating ionic polarization.35–38) Therefore, we think that the substitutional effect of nitrogen will be clarified by combined first-principles calculation and THz dielectric measurement.
4. Conclusions
In this study, N-doped SrTiO3 single crystals were fabricated by annealing SrTiO3 single crystals in NH3 gas. The nitrided SrTiO3 single crystals were yellow corresponding to the narrowing of the band gap. The N-doped SrTiO3 was assumed to have the chemical composition SrTiO3−3xN2x, which contained oxygen vacancies. Since oxygen vacancies might reduce mechanical strength and dielectric properties, a SrTiO3 crystal co-doped with nitrogen and niobium (SrTiO3:N,Nb) were fabricated to reduce the number of oxygen vacancies. The semiconducting SrTiO3:Nb crystal changed to a dielectric SrTiO3:N,Nb crystal with a resistivity of 6 × 1012 Ω·cm with annealing in NH3 gas. Furthermore, XPS measurement indicated that niobium doping was effective for increasing the amount of doped nitrogen. The dielectric permittivity increased with the amount of doped nitrogen, showing the effectivity of nitrogen doping for enhancing the dielectric permittivity by nitrogen doping in perovskite oxides. In further study, the substitutional effect of nitrogen should be clarified by combined first-principles calculation and THz dielectric measurement.
Acknowledgement
This work was supported by JSPS KAKENHI Grant Number 26420676.