Abstract
The invasive potential of cancer cells strongly depends on cellular stiffness, a physical quantity that is not only regulated by the mechanical impact of the cytoskeleton but also influenced by the membrane rigidity. To analyze the specific role of membrane rigidity in cancer progression, we treated cancer cells with the Acetyl-CoA carboxylase inhibitor Soraphen A and revealed an alteration of the phospholipidome via mass spectrometry. Migration, invasion, and cell death assays were employed to relate this alteration to functional consequences, and a decrease of migration and invasion without significant impact on cell death has been recorded. Fourier fluctuation analysis of giant plasma membrane vesicles showed that Soraphen A increases membrane rigidity of carcinoma cell membranes. Mechanical measurements of the creep deformation response of whole intact cells were performed using the optical stretcher. The increase in membrane rigidity was observed in one cell line without changing the creep deformation response indicating no restructuring of the cytoskeleton. These data indicate that the increase of membrane rigidity alone is sufficient to inhibit invasiveness of cancer cells, thus disclosing the eminent role of membrane rigidity in migratory processes.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Cancer cells differ significantly in phospholipid composition compared to their non-malignant counter parts [1, 2] and changes in membrane lipid composition are strongly connected to alteration in bending rigidity [3]. Decoding cell mechanics of cancer is necessary to understand it from a biomechanical perspective [4, 5]. However, current research is mostly focused on specific molecular and biochemical targets [6, 7] whereas research on a more general level, such as mechanical features, is still an emerging field [8–10]. Identifying mechanical properties of cells and their membranes that affect migratory processes [11] is key to understanding cancer progression [9]. Beyond that, it opens the possibility of targeting and manipulating the physical characteristics of membranes. Membrane affecting drugs exist, for example, as anesthetics [12] and against diseases of the vascular system, such as hypertension [13]. Lipid components had a significant influence on motility and therefore play a role in cancer progression [2].
In the present study we characterize the impact of Soraphen A on plasma membrane rigidity and migratory and invasive properties of cancer cells. Soraphen A inhibits the key enzyme in the fatty acid metabolism, the Acetyl-CoA carboxylase (ACC1). ACC1 catalyzes the ATP-dependent carboxylation of acetyl-CoA to produce malonyl-CoA, which is the first committed step in fatty acid synthesis. It has been shown that inhibition of ACC1 by Soraphen A interferes with fatty acid elongation resulting in an increased level of polyunsaturated fatty acid species in prostate, breast and colorectal cancer cells and is a tool to reverse the lipogenic phenotype in cancer cells [14]. It was further reported that Soraphen A decreases the content of mono-unsaturated and saturated acyl chains [15]. Alterations in fatty acid content of cells affect several cellular processes, such as cell signaling and gene expression, and thus influence the progression of diseases like cancer [15, 16]. While the functional consequences of ACC1 inhibition have been analyzed with regard to cellular growth and apoptosis thus far [17, 18], this study focuses on the effects of Soraphen A on membrane composition, mechanical characteristics, and hence, migratory processes of highly aggressive cancer cells.
2. Materials and methods
2.1. Cell culture and compounds
Soraphen A was isolated by Gerth et al as described previously [19] and dissolved in ethanol. The invasive mammary carcinoma cell line MDA-MB-231 was obtained from Cell Line Services (Eppelheim, Germany) and cultured in DMEM media (PAA, Coelbe, Germany) supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (P/S). T24 bladder carcinoma cells were kindly provided by Barbara Mayer (Department of Surgery, University of Munich, Germany) and recently authenticated by the DSMZ (Braunschweig, Germany). T24 cells were maintained in McCoy's 5A medium (PAA) supplemented with 10% FCS, 2 mM L-glutamine and 1% (P/S).
2.2. Analysis of phospholipids by liquid chromatography ESI tandem mass spectrometry
Phospholipids were extracted, separated by reversed phase liquid chromatography and detected by ESI tandem mass spectrometry as described in [20]. In brief, water, methanol, chloroform and saline (final ratio: 14:34:35:17) were successively added to the cells. The organic layer was evaporated and the residual dissolved and diluted in methanol. The extracted phospholipids were separated on an Acquity UPLC BEH C8 column (1.7 μm, 1 × 100 mm, Milford, MA) using an AcquityTM Ultraperformance LC system (Waters, Milford, MA). The chromatography system was coupled to a QTRAP 5500 Mass Spectrometer (AB Sciex, Darmstadt, Germany) with an electrospray ionization source. Both fatty acid anion fragments were detected through multiple reaction monitoring. The most intensive transition was selected for quantification. Mass spectra were processed using Analyst 1.6 (AB Sciex, Darmstadt, Germany). The reported method was optimized to compare phospholipid profiles between samples but not for absolute quantification.
2.3. Optical Stretcher measurements
Cells were detached from flask and re-suspended in culture medium. Cell suspension in tube was connected to the optical stretcher setup [21], a dual beam fiber laser trap (figure 1(a)). Whole deformation measurement was recorded as an image series (30 fps). The microscope stage temperature was held constant at 23 °C. A minimum of 300 cells were recorded for each experiment. Cell numbers used for analysis after sorting out rotating cells are shown in figures 4(c) and (d).
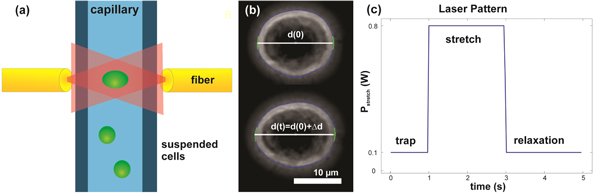
Figure 1. Optical stretcher setup. (a) Cells are pumped to the focus of the optical trap. After a cell gets trapped it is stretched with 800 mW and the increased diameter is recorded with an edge detection algorithm (b). The complete measurement pattern of a single deformation experiment is shown in (c).
Download figure:
Standard image High-resolution imageAn edge detection Matlab algorithm (The MathWorks Inc., Natick) constructs deformation data from the images and corrects for small angle rotations in the trap (figure 1(b)). Relative deformation  is the ratio of elongation and initial cell diameter d0 along laser axis,
is the ratio of elongation and initial cell diameter d0 along laser axis,  = (d(t)−d0)/d0, and plots are shown as creep deformation J(t) =
= (d(t)−d0)/d0, and plots are shown as creep deformation J(t) =  /σ0, with σ0 being the optically induced stress linearly dependent on the applied laser power PStretch (figure 1(c)) [22, 23]. Creep deformation J is shown as median, appropriate for the non-Gaussian distribution of the data, and bootstrapping was used to estimate a 95% confidence interval. Additionally, a two-sample Kolmogorov–Smirnov test was used to control if the data are from different distributions at the end of the stretch phase.
/σ0, with σ0 being the optically induced stress linearly dependent on the applied laser power PStretch (figure 1(c)) [22, 23]. Creep deformation J is shown as median, appropriate for the non-Gaussian distribution of the data, and bootstrapping was used to estimate a 95% confidence interval. Additionally, a two-sample Kolmogorov–Smirnov test was used to control if the data are from different distributions at the end of the stretch phase.
2.4. Giant plasma membrane vesicles (GPMVs)
Prior to vesiculation, cells were grown to 90% confluence in 75 cm2 tissue flasks. The culture medium was removed and rinsed with a buffer solution composed of 150 mM NaCl (Sigma-Aldrich, S7653), 10 mM 2-(4-(2-hydroxyethyl)-1-piperazinyl)-ethanesulfonic acid (HEPES, Sigma-Aldrich, St.Louis, MO, USA, H3375) and 2 mM CaCl2 (Sigma-Aldrich, C5080). Buffer ingredients, di-thiothreitol (DTT) (Roth, 6908) and paraformaldehyde (PFA) (Sigma-Aldrich, P6148) powder were dissolved in ultra-pure water (Milli-Q system Integral 5, Merck Millipore, Billerica, MA, USA, R > 18 MΩ cm). Afterwards, cells were incubated in a shaker (37 °C, 5% CO2 and 60 cycle min−1) for 120 min in 2.5 ml buffer, containing 25 mM PFA and 4 mM DTT. After shaking, the content was settled on ice for about 45 min. In order to observe GPMVs, the upper 3/4 of the solution was pipetted on an object plate for imaging (optical phase contrast microscope, 100× oil immersion objective, LEICA DM IRB).
2.5. Fourier analysis of thermally excited membrane fluctuations
The theoretical background of the fluctuation analysis was introduced by Helfrich in 1973 [24]. He proposed the curvature energy per unit area of bilayers:
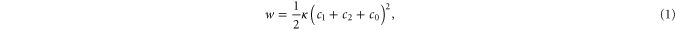
where c0 is the spontaneous curvature, κ the bending rigidity and c1 and c2 principle curvatures. More than 10 years later, Schneider extended Helfrich's model to quasi-spherical vesicles [25]. Schneider described thermally excited membrane fluctuations using displacements u(θ, φ, t) of a vesicle from its spherical form and decomposed them in spherical harmonic eigenfunctions Ylq(θ, φ). The radius of the vesicle can be expressed as:
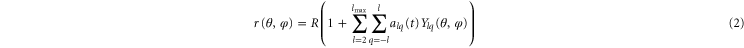
alq(t) are time-dependent amplitudes, θ the polar angle, φ the azimuthal angle, l the azimuthal, and q the magnetic quantum number. R describes the averaged radius of the sphere. States with l < 2 correspond to spherical translations and violate volume conservation. Provided the thermally excited modes follow the equipartition theorem and the vesicle is quasi-spherical, dimensionless mean square amplitudes alq from each spherical harmonic can be expressed as:
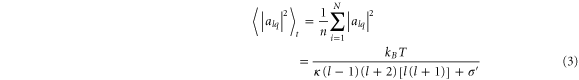
T denotes the temperature, kB the Boltzmann constant, and σ' = σeff R2/κ introduces the effective tension. The expected value is calculated as ensemble average.
The analysis of thermally excited membrane fluctuations is based on sequences of snapshots obtained by optical microscopy. A representative ensemble of n images (≈10 000) per vesicle was recorded with an iXon camera (Andor, UK) while the vesicles were observed by phase contrast microscopy. We worked with acquisition times of about 1 min and rates between 90 and 150 frames per second to guarantee that vesicles were able to constitute most of its available configurations. For typical giant vesicles 1 min is much longer than the recurrence or the relaxation times of the excitation [26].
Equation (3) depends only on l, which allows studying membrane fluctuations by observing just one plane of the vesicle. In the experimental setup we obtained information from the equatorial plane only (θ = π/2). In order to detect the vesicles contour R(φ), a Matlab (The MathWorks Inc., Natick) gradient based edge detection algorithm with subpixel resolution [21] was used. The shape of each single vesicle is described with 256 radii R(φi). φi denotes the azimuthal angle in the image plane. Fourier analysis of the observed 2D vesicle contour fluctuation was correlated to Schneider's 3D theory [27, 28]. The relative deformations u(φ, t) of the vesicle can be expressed as amplitudes Vq(t) in the equatorial plane using Fourier transformation:
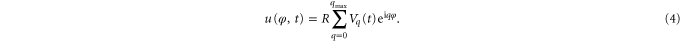
The mean square values of the amplitudes Vq(t) were obtained from equations (2) and (4):
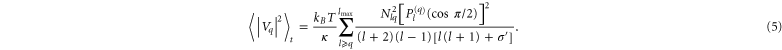
 was estimated by averaging Vq over the number of taken images n per vesicle.
was estimated by averaging Vq over the number of taken images n per vesicle.  (cos(π/2)) denote the fully normalized associated Legendre functions in the image plane and q the mode number. We realized the Fourier transformation from equation (4) with a discrete transformation to obtain discrete coefficients. The measured mean square values can be introduced in equation (5) to obtain κ and σeff by a two-parameter fit. The sum in equation (5) is rapidly converging for moderate tensions [28].
(cos(π/2)) denote the fully normalized associated Legendre functions in the image plane and q the mode number. We realized the Fourier transformation from equation (4) with a discrete transformation to obtain discrete coefficients. The measured mean square values can be introduced in equation (5) to obtain κ and σeff by a two-parameter fit. The sum in equation (5) is rapidly converging for moderate tensions [28].
As seen from equation (5) the effective tension σeff dominates the fluctuations for low-wave-number modes which are typically ignored in the fluctuation analysis [28]. These modes (q < 7) lead to systematically too high bending rigidities. The tension dominated regime is followed by an intermediate one used to calculate the bending rigidity. In this regime, κ is practically independent of the wavenumber and mean square amplitude of the contour fluctuations  scales as
scales as  [29]. High-wavenumber modes (q ≥ 16) are noise dominated and were also ignored. In the present work we used vesicles with observable flicker and only modes 7 ≤ q < 16 to determine the bending rigidity. The fits in figure S1 show the typical behavior of the bending dominated regime (
[29]. High-wavenumber modes (q ≥ 16) are noise dominated and were also ignored. In the present work we used vesicles with observable flicker and only modes 7 ≤ q < 16 to determine the bending rigidity. The fits in figure S1 show the typical behavior of the bending dominated regime ( for the analyzed modes in agreement with [29, 30].
for the analyzed modes in agreement with [29, 30].
Engelhardt further suggested a simplification for practically tension free vesicles [27]:
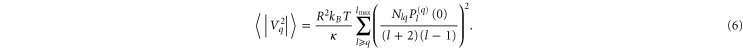
R denotes the average radius of each vesicle obtained from all images n per dataset. A two-sample Kolmogorov–Smirnov test was performed to control if the data are from different distributions.
2.6. Migration and invasion assay
2 × 105 MDA-MB-231 or T24 cells, respectively, were seeded on a six-well plate and stimulated with Soraphen A for 2 h. For MDA-MB-231 Boyden chambers with 8 μm inserts and for T24 cells 5 μm inserts were used. The migration time was 4 (MDA-MB-231) or 16 h (T24), respectively. The invasion time was 24 h for both cell lines. Non-migratory or non-invading cells were removed from the upper insert compartment with a cotton bud. Migrating and invading cells, respectively, were visualized by crystal violet staining and counted.
2.7. Cell death assay
Cell death rate was determined with propidium iodide (PI)—exclusion assays. In brief, cells were treated for 24 h with increasing concentration of Soraphen A, washed, stained with 50 μg ml−1 Propidium iodide, and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson, Heidelberg, Germany).
2.8. Statistical analysis
Invasion and migration assays were performed three times. Data are expressed as means ±S.E.M and analyzed using one-way-ANOVA +Tukey HSD post hoc or Student's t test. Values of p < 0.05 were considered as significant.
3. Results
3.1. Mass spectrometry measurements
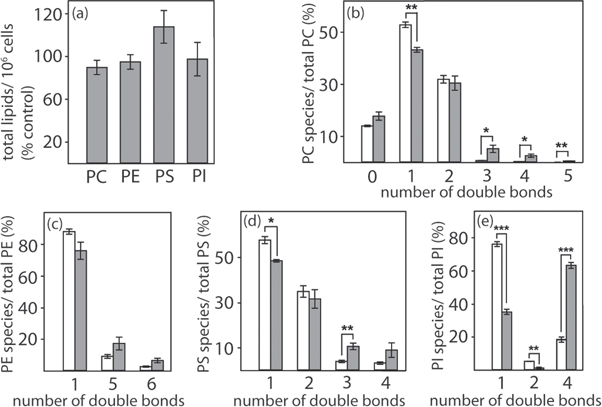
To investigate whether modulations of lipid composition of plasma membranes influence the biomechanical properties of cancer cells and thus their metastatic potential, in the first step, the breast cancer cell line MDA-MB-231 was treated for 6 h with the ACC1 inhibitor Soraphen A, and a detailed lipidome analysis was performed with ESI tandem mass spectrometry. Extending first reports regarding the effect of Soraphen A on the phospholipid content after long incubation times of 72 h [15], the ACC inhibitor does not alter the total phospholipid content (measured as sum of all species detected). One exception was phosphatidylserine (PS), whose levels were slightly but non-significantly increased (figure 2(a)). Of note, desaturation in all analyzed phospholipid species increased after treatment with Soraphen A (figures 2(b)–(e)).
Figure 2. Effect of Soraphen A on the phospholipid composition of MDA-MB-231 cells. Cells (5 × 106) were treated with vehicle only (ethanol; (b)–(e) white bars) or Soraphen A (100 nM; (a)–(e) dark bars) for 6 h. Extracted phospholipids were analyzed by liquid chromatography ESI tandem mass spectrometry. (a) Total signal intensities of phospholipid subclasses in Soraphen A-treated cells. Total phospholipid subclass intensities combine the intensities of all species of the respective subclass and were normalized to the number of cells. 100% corresponds to the signal intensities of vehicle-treated cells and is based on relative units. (b)–(e) Distribution of phospholipid species depending on their desaturation. Summarized signal intensities are given relative to the total phospholipid subclass intensity. (*p < 0.05, ** p < 0.01, *** p < 0.001.)
Download figure:
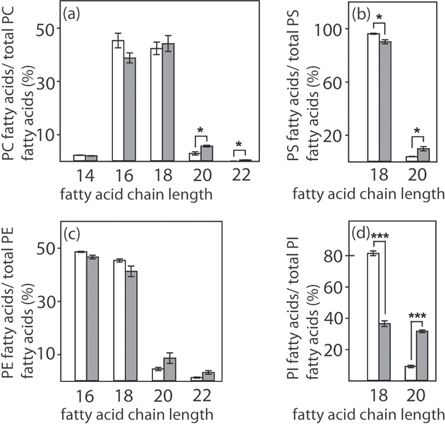
Standard image High-resolution imageHowever, the composition of membrane phospholipids, including phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylserines (PS), and phosphatidylinositols (PI) is strongly altered by Soraphen A treatment after the short treatment time of only 6 h (figures 3(a)–(d)). The proportion of phospholipids is shifted towards those with longer fatty acid chains, whereas the proportion of phospholipids with shorter fatty acid chains significantly declined after incubation with Soraphen A.
Figure 3. Effect of Soraphen A on the phospholipid composition of MDA-MB-231 cells. Cells (5 × 106) were treated with vehicle only (ethanol; white bars) or Soraphen A (100 nM; dark bars) for 6 h. Extracted phospholipids were analyzed by liquid chromatography ESI tandem mass spectrometry. (a)–(d) Distribution of phospholipid species depending on their fatty acid chain length (Student's t-test, * p < 0.05, *** p < 0.001). The proportion of phospholipid species (=relative intensity) is given as a percentage of the sum of all species in the respective subclass (=100%).
Download figure:
Standard image High-resolution image3.2. Biomechanical behavior of cytoskeleton and membrane
Since phospholipids are the main components of cellular membranes, we assumed that Soraphen A induced changes in phospholipid composition might affect rigidity of membranes and deformation of whole cells. Hence, by evaluating membrane fluctuations [31], bending rigidity of cellular membranes was determined as described in [27, 32]. In order to exclude effects such as cytoskeletal restrains, membrane rigidity was determined using GPMVs [33]. GPMVs are derived from viable cells by vesiculation—an energy, pH, and temperature-dependent procedure. The process is chemically induced by PFA and potentiated with mono- and divalent cations as well as 1,4-di-thiothreitol (DTT) [33, 34]. A contraction of the cytoskeleton induces an increased hydrostatic pressure inside the cell and pieces of cellular membrane separate from the cytoskeleton [35]. It should be noted, that cellular membranes in living cells are dynamic systems where cell signaling, exo-, and endocytosis constantly occur, and it is reported that those processes can also be affected by membrane mechanics [36, 37]. To generate GPMVs, the cell membrane is decoupled from the underlying cytoskeleton during vesiculation, which also disrupts these fundamental cellular processes and may inhibit some membrane enzymes [38]. We cannot exclude that these processes are influenced during vesiculation and thus have an impact on bending rigidity. Nevertheless, cells are not homogenized during vesiculation and GPMVs are representatives of the cell surface [38]. Most of the observed vesicles had radii between 5 and 15 μm. GPMVs contain numerous membrane lipids and polypeptides in contrast to artificially biomimetic membranes and are devoid of cortical actin assembly [34]. Hypotonic lysis removes cytosolic ingredients. This allows interaction-free measurement of rigidity of cellular membranes via Fourier analysis of thermal vesicle shape fluctuation. Fourier analysis or flicker spectroscopy of the fluctuating vesicle contour is a sensitive method to estimate the bending rigidity κ of thermally excited vesicle membranes. The aggressive cancer cells lines MDA-MB-231 (breast cancer cells) and T24 (urinary bladder carcinoma cells) were incubated with 1 μM Soraphen A for 2 h. 45 treated and 48 untreated vesicles obtained from MDA-MB-231 cells were measured and 30 treated and 28 untreated vesicles for T24 cells, respectively. Notably, Soraphen A did not apparently affect the efficiency of the vesiculation process.
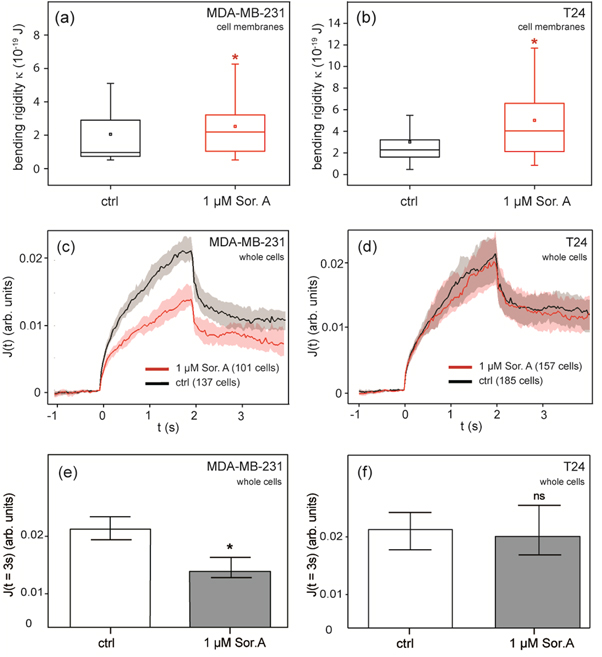
As presented in figures 4(a) and (b), bending rigidity of cell membranes obtained from Soraphen A treated MDA-MB-231 cells (κ = 2.2 × 10−19 J) showed an increased median compared to untreated GPMVs (κ = 1.0 × 10−19 J). The same behavior was observed for membranes obtained from T24 cells (κ = 4.4 × 10−19 J for treated and κ = 2.3 × 10−19 J for untreated vesicles). The results indicate that the inhibition of ACC1 and the resulting modulation of phospholipid composition enhances the rigidity of cellular membranes.
Figure 4. Biomechanical behavior of human carcinoma cells and their plasma membranes after Soraphen A treatment. Boxplots of bending elastic moduli κ of cell membranes (GPMVs) obtained from the mammary carcinoma cell line MDA-MB-231 (a) and the bladder carcinoma cell line T24 (b). Both show the impact of 1 μM Soraphen A on cell membrane rigidity, displaying upper quartile, median, mean value, lower quartile and a 10–90% whisker range for κ (Kolmogorov–Smirnov test, * p < 0.05). Median relative deformation of whole MDA-MB-231 cells (c) and T24 cells (d) in optical stretcher measurements plotted over time with 800 mW stretch phase from 0 to 2 s. (e), (f) Comparison of optical stretcher measurements at the end of the stretch phase (Kolmogorov–Smirnov test, * p < 0.05, ns: not significant).
Download figure:
Standard image High-resolution imageIn addition, optical stretcher measurements were performed to determine whole cell deformation and thus stiffness of cells in the presence of an intact cytoskeleton [21]. Cells were pumped in a microfluidic system, serially stopped in the focus of the phase contrast microscope, and trapped by the laser with 100 mW. A single cell deformation measurement is composed of a 1 s trap of the cell, following a 2 s stretch phase with a laser power of 800 mW, and another trap phase for 2 s to record relaxation. The setup is illustrated in figure 1(a). As shown in figure 4(c), cellular stiffness of MDA-MB-231 cells was increased after incubation with 1 μM Soraphen A for 2 h. 101 treated and 137 control cells were measured. In contrast, the ACC1 inhibitor did not alter median creep deformation J of T24 cells (157 treated and 185 control cells) (figure 4(d)). The median of the creep deformation J was analyzed at the end of the stretch phase (t = 3 s) as most significant parameter (figures 4(e) and (f)). Soraphen A has affected membrane rigidity and cytoskeleton in the breast epithelial cell line, where as in the urinary bladder cell line only membrane rigidity was affected. The stage of the optical stretcher is held at 23 °C during all measurements. The optical stretcher heats the cells inside the capillary during the measurement up to 44 °C for 2 s in the center of the trap [39, 40]. Although we cannot exclude phase transition induced changes in bending rigidity of whole cell membranes during the stretching process, this should not have an impact on the measured creep deformation. This is because the contribution of membrane rigidity to creep deformation is significantly lower compared to the underlying intact cytoskeleton in these whole cell experiments.
3.3. Migration and invasion behavior
To investigate whether Soraphen A has a functional impact on metastatic potential of cancer cells, Boyden chamber assays were performed. Soraphen treated cells were detached and plated into uncoated (for analysis of migration) or matrigel coated (for invasion assays) Boyden chamber inserts and allowed to migrate or invade towards a 10% FCS + 100 ng ml−1 EGF gradient. Despite the different behavior of T24 and MDA-MB-231 cells in the optical stretcher experiments, Soraphen A inhibits migration and invasion capacity of both cell lines to a similar extent (figures 5(a)–(d)) without significantly affecting cell death (figure 6). The strongest effect of Soraphen A on cell invasion and migration was observed for cells treated with 1 μM Soraphen A.
Figure 5. Effect of Soraphen A on migration and invasion. Cells were treated with increasing Soraphen A concentrations for 2 h. Migration of (a) MDA-MB-231 and (b) T24 cells was analyzed by a Boyden chamber migration assay. To analyze invasion of cells a modified Boyden chamber assay for (c) MDA-MB-231 and (d) T24 cells was performed. (* p < 0.05, ** p < 0.01, *** p < 0.001.)
Download figure:
Standard image High-resolution imageFigure 6. Influence of Soraphen A on cell death of human epithelial carcinoma cells. Treatment of MDA-MB-231 (a) and T24 (b) cells with Soraphen A for 24 h did not affect cell death.
Download figure:
Standard image High-resolution image4. Discussion
This study demonstrates that Soraphen A, as an ACC1 inhibitor, changed the phospholipid composition towards a higher chain length and polyunsaturated lipid species. Further, Soraphen A hinders cancer cell migration as well as invasion and alters the mechanical properties of the plasma membrane. To distinguish influences of cytoskeleton and plasma membrane on mechanical properties, optical stretcher measurements of whole cells were performed in addition to fluctuation assays of GPMVs. This allows an interpretation regarding the role of membranes in the context of migration.
Mass spectrometry measurements of the lipid composition in cancer cells treated with Soraphen A suggest a molecular explanation of the changed biomechanical properties resulting in increased membrane rigidity. In contrast to the study of Jump et al showing an inhibition of fatty acid elongation in HepG2 hepatocellular carcinoma cells, we were able to demonstrate that the amount of fatty acids with higher chain lengths increases in a broad range of phospholipids after Soraphen A treatment [14]. This may be due to the different cell lines used in the studies or depend on the distinct experimental setup, the varying incubation times, and concentrations of Soraphen A. However, it is well known that an increment in fatty acid chain length results in a higher membrane rigidity due to enhanced Van der Waals interactions [41]. The phospholipid content of the whole cell, including not only lipids from the cell membrane but also from intracellular sources, was analyzed. Since changes in fatty acid biosynthesis are likely to be distributed over all cellular membranes (due to phospholipid remodeling by lysophospholipid acyltransferases and phospholipases A2 [42]), our data cannot exclude that Soraphen A affects the phospholipid content of intracellular membranes and thus the migration behavior. In this study, treatment with the ACC1 inhibitor resulted in an upregulation of phospholipids with double bonds. Although it is described in the literature that enhanced levels of polyunsaturated fatty acids hinder dense packing of the membrane [43] and thus decrease their rigidity, this study indicates that the acyl chain length might dominate plasma membrane rigidity.
Fourier fluctuation analysis demonstrates that GPMVs of Soraphen A treated MDA-MB 231 and T24 cells have a higher rigidity compared to untreated cells. This may be caused by the phospholipid composition shift towards those with longer chain lengths. The absolute values of the measured bending rigidities κ (figures 4(a) and (b)) are in a range of values measured for vesicles composed of a few synthetic lipids [28, 44]. The observed bending rigidities in literature vary strongly depending on membrane composition. For example, κ = 10−19 J for a ternary mixture of DOPC, SM, and Cholesterol (70:10:20) and κ = 7×10−19 for a binary mixture of SM and Cholesterol (80:20) [28]. The differences between the bending rigidity of Soraphen A treated and untreated control cells are in a similar range as formerly reported effects of cholesterol on specific artificial vesicles [28, 45]. However, GPMVs used in this study are plasma membrane blebs without cortical actin assembly. They contain a large number of lipids and peptides, whereas most of the studied artificial vesicles contain only two or three different lipids and their composition has to be distinguished from GPMVs.
The cellular stiffness of whole MDA-MB-231 cells increased after incubation with Soraphen A, whereas the ACC inhibitor did not alter the stiffness of T24 cells. Of note, the reported differences in biomechanical properties between Soraphen A treated and untreated cells, measured with the optical stretcher, may not just depend on lipid composition but may also be caused by suppression of other cellular processes such as endo- and exocytosis. The combination of stretcher measurements on cancer cells and fluctuation analyses of their GPMVs suggest that membrane rigidity alone is affected by Soraphen A.
The presented results of Soraphen A induced weakened migration and invasion, analyzed via Boyden chamber assays, support the idea that membrane rigidity is correlated with a change in migratory capacity. We assume that modulating membrane rigidity only, even without affecting stiffness of the whole cell, has an impact on cancer cell movement. In this case, increased membrane rigidity would be sufficient to hinder cell motility.
Our statement that lower membrane rigidity correlates with higher migration potential is supported by a study about primary cancer cells isolated from human patients [47]. Primary cancer cells contain higher levels of phospholipids with shorter fatty acid chain length than non-malignant primary samples, resulting in softening of the cell membrane. This indicates the possible reversal of the malignant phenotype with Soraphen A by influencing either rigidity or migration.
In summary, modulating membrane rigidity and cell stiffness with the chemical compound Soraphen A allowed us to investigate these important mechanical features for the first time with respect to migration and invasion. Membrane rigidity measurements based on Fourier fluctuation analysis have only been applied to vesicles composed of a few synthetic lipids and red blood cells thus far [3, 32]. In this study GPMVs with a more physiological membrane composition were used. These results point to the important role of membrane rigidity in migratory processes, even with unaffected cell stiffness, and suggest that membrane rigidity is more closely related to cancer progression than previously assumed [46]. Thus, targeting membrane features of cancer cells offers new therapeutic perspectives in membrane research and possibly cancer biology.
Acknowledgments
Graduate student Sebastian Schmidt (Project KA1116/7-1) was financed by the DFG. We would like to thank BuildMoNa for supporting Chris Händel. Further we would like to thank Professor Dr Ana-Suncana Smith, Daniel Schmidt and Jörg Schnauß for fruitful discussion. This work was further supported by Nanosystems Initiative Munich (NIM) (Katharina Stoiber, Angelika M Vollmar) and DFG research grant FOR1406 (Rolf Müller, Angelika M Vollmar, Oliver Werz). We would like to thank Patricia Warne and Laura-Kaisa Maijala for proofreading.