Abstract
The large marine snow formation event observed in oil-contaminated surface waters of the Gulf of Mexico (GoM) after the Deepwater Horizon accident possibly played a key role in the fate of the surface oil. We characterized the unusually large and mucus-rich marine snow that formed and conducted roller table experiments to investigate their formation mechanisms. Once marine snow lost its buoyancy, its sinking velocity, porosity and excess density were then similar to those of diatom or miscellaneous aggregates. The hydrated density of the component particles of the marine snow from the GoM was remarkably variable, suggesting a wide variety of component types. Our experiments suggest that the marine snow appearing at the surface after the oil spill was formed through the interaction of three mechanisms: (1) production of mucous webs through the activities of bacterial oil-degraders associated with the floating oil layer; (2) production of oily particulate matter through interactions of oil components with suspended matter and their coagulation; and (3) coagulation of phytoplankton with oil droplets incorporated into aggregates. Marine snow formed in some, but not all, experiments with water from the subsurface plume of dissolved hydrocarbons, emphasizing the complexity of the conditions leading to the formation of marine snow in oil-contaminated seawater at depth.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Marine snow is defined as particles >0.5 mm that consist of many smaller, organic and inorganic particles including bacteria, phytoplankton, feces, feeding structures, detritus and bio-minerals (Alldredge and Silver 1988, Thornton 2002, Simon et al 2002). Marine snow is ubiquitous in the ocean and is responsible for the majority of the downward transport of material by gravitational settling, because only such large particles sink at velocities (100s meters per day) sufficient to escape complete degradation within the upper ocean. To date, two formation mechanisms have been described for marine snow: physical coagulation where component particles collide and remain attached (true aggregates) and zooplankton activity which produces large feces or feeding structures like pteropod webs or appendicularian houses (Alldredge and Silver 1988).
Marine snow formation due to coagulation depends on particle sizes and the concentrations of particles like cells, feces or minerals, as well as on their stickiness and the processes that increase particle collision rates, e.g. turbulence or differential settling (Jackson 1990, Burd and Jackson 2009). Because coagulation requires a critical particle concentration (Jackson 2005), it is most commonly observed at the peak of large diatom blooms. Many phytoplankton taxa release exopolymeric substances (EPS) into the water (Myklestad 1995) that enhance the aggregation process. These exopolymers slough off cell surfaces as nanofibers (Leppard 1997) which self-assemble to colloidal sized nanogels (Chin et al 1998), which coalesce to form larger gels and porous networks, many of which become visible as μm to mm sized transparent exopolymeric particles (TEP; Passow 2000, Verdugo and Santschi 2010). TEP are sticky particles that enable aggregation by providing the glue and the matrix of phytoplankton aggregates (Passow et al 1994).
In most oceans, marine snow varies in size from millimeters to centimeters, but larger extremely mucus-rich marine snow episodically forms in the Adriatic and Tyrrhenian Sea, reaching up to 3 m in diameter (Giani et al 2005). The formation mechanisms and the conditions that lead to the formation of such huge marine snow in the Mediterranean are still under debate, although their formation often correlates with specific physical conditions (water column stratification, low mixing) and biological production patterns in the water column (nutrient dynamics, microbial production of sticky TEP and other EPS; e.g. Giani et al 2005 and references therein). When sinking to the seafloor, these very large marine snow particles may cover and suffocate benthic communities, causing anoxia and generally impacting the benthic communities as well as the pelagic ecosystem and fishing efforts in the Adriatic and Tyrrhenian Sea (Stachowitsch et al 1990, Herndl 1992, Penna et al 1993, Herndl et al 1999, Mecozzi et al 2004).
Mucus-rich marine snow, morphologically similar to that observed in the Mediterranean, has also been observed in the Gulf of Mexico (GoM) shortly after the onset of the Deepwater Horizon (DWH) oil spill in May 2010. Large (cm sized), mucous-rich marine snow was seen floating at the surface in the immediate vicinity of oil layers near the sunken platform. We suggest that this marine snow formed in situ due to the presence of oil and will hereafter refer to it as 'GoM-snow'. Another month later, in June, all GoM-snow had vanished from view and its rapid sedimentation to depth was hypothesized (Dell'Amore 2010).
Marine snow formation in oil-contaminated surface waters has been described before during the Ixtoc oil spill in the GoM in 1979 (Patton et al 1981), and was implicated by the Federal Interagency Solution Group as being a leading cause for the transport of the spilled oil to the seafloor (Federal Interagency Solution group: Oil Budget Calculator Science and Engineering team 2010). Despite their apparent role in the fate of oil in the ocean, little is known about the formation mechanisms of marine snow in the presence of oil. It is also unclear if the formation of marine snow is restricted to oil-contaminated surface waters or whether this mechanism is active within the water column. This question becomes relevant in the discussion about the fate of spilled DWH oil and gas that formed large subsurface plumes. These plumes, characterized by fluorescence, transmission and oxygen signals consistent with the presence of oil droplets were first observed in May and June between 1000 and 1400 m depths southwest of the wellhead site (Camilli et al 2010, Diercks et al 2010, Hazen et al 2010). Later subsurface plumes were also discovered northeast of the spill site, towards DeSoto Canyon. Bacteria with genes for hydrocarbon degradation were clearly enriched within the plumes and were possibly responsible for the disappearance of the biodegradable fraction of the oil-contaminants (Hazen et al 2010, Valentine et al 2010, Kessler et al 2011, Redmond and Valentine 2011, Lu et al 2012). By August many of the subsurface plumes were much less distinct, although unusual features in oxygen, fluorescence and beam attenuation still reflected their (past) presence (unpubl.).
The goal of our project is to better understand the formation mechanisms of marine snow in the presence of oil as well as the role of GoM-snow in the cycling of oil-derived carbon within pelagic ecosystems. Here we characterize marine snow collected at the surface near the DWH wellhead in May 2010 and present experimental data elucidating the different formation mechanisms of such marine snow. We conducted roller table experiments to investigate the potential of seawater from unaffected sites or from the deep plumes to form marine snow either in the presence or absence of pristine or weathered oil. These experiments are complicated by the fact that methods based on filtration (e.g. onto 0.4 μm filters) or on colorimetric analysis cannot be used at oil concentrations >0.01%. Based on the results from our experiments we propose three mechanisms by which hydrocarbons promote the formation of marine snow.
2. Materials and methods
2.1. Characteristics of marine snow collected from oil-contaminated surface waters
Natural mucous-rich marine snow floating at the sea surface in direct association with a visible oil layer near the DWH spill site was collected by bucket during the first oil spill response cruise onboard RV Pelican at two different sites on 11 May 2010 (28°42.150'N 88°21.729'W and 28°41.988'N 88°16.760'W; figure 1). Marine snow was carefully transferred into glass scintillation vials and stored under cool and dark conditions for about one month until further processing at the laboratory at UC Santa Barbara. Marine snow from five replicate vials was described and used for measurements of either its sinking velocity (8 each from a total of 4 vials) or the solid hydrated density of its constituents (3 from 1 vial). Sizes and sinking velocities of 32 individual marine snow particles were measured under a dissecting scope and in a settling column (Ploug et al 2010). The excess density of each marine snow particle was calculated from its sinking velocity and its equivalent spherical diameter (ESD) using the Navier–Stokes drag equation as described in Iversen and Ploug (2010). The solid hydrated density (ρs, g cm−3) of the components making up the marine snow was determined in a density gradient (Gaerdes et al 2011). Six dilutions ranging in density from 1.08 to 1.34 g cm−3 were made using Ludox TM colloidal silica, sucrose and distilled water. All solutions were buffered to pH 8.1 with 0.0125 M Tris plus 0.0125 M Tris-HCl (final concentration) and were iso-osmotic with seawater (Gaerdes et al 2011). To facilitate visual analysis, a dye was added to every second layer. Two ml of each dilution were gently layered in a 20 ml centrifuge tube which was stored at 5 °C overnight and warmed to room temperature before use. One ml of sterile seawater was gently applied on top of the density gradient and a single marine snow particle transferred to each tube, letting it settle into the seawater layer. Next, samples were centrifuged at 3000 rpm for 30 min and all layers containing particles were removed from the tube and weighed using a Mettler Toledo fine balance. Particle numbers in each layer were estimated using a dissecting microscope and a weighted average solid hydrated density of each marine snow particle calculated based on the measured densities and abundances of particles. The porosity of the collected marine snow was estimated by assuming that all non-solid components have the density of seawater and comparing the average excess solid hydrated density of the components with the excess density of the whole marine snow particle. As marine snow structure is known to change due to handling or upon contact with solid surfaces (Ploug and Jorgensen 1999) our measurements most likely do not reflect in situ conditions accurately. However, they reveal useful characteristics in comparison with other types of marine snow.
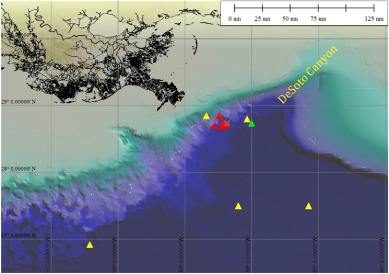
Figure 1. Sampling sites of source water collected for experiments during cruise I on RV Pelican in May (green-gray triangle), cruise II on RV Walton Smith in June (dark red triangles) and cruise III on RV Oceanus in August (light yellow triangles). The collection sites of the GoM-oil (28°44'N; 88°22'W) and the GoM-snow (28°42'N; 88°22'W and 28°42'N; 88°17'W) are covered by the cross symbolizing the Deep Horizon well head. See also table 1 for positions.
Download figure:
Standard image2.2. Marine snow formation experiments
A series of roller table experiments was conducted to investigate the mechanisms leading to the formation of marine snow in the aftermath of the oil spill. In each case, snow free water was collected and incubated on roller tables, which simulate a natural environment that encourages marine snow formation (Shanks and Edmondson 1989). Different source waters for these experiments were collected during three oil spill response cruises between May and August 2010 in a variety of geographic locations (figure 1) using Niskin or Go-flow bottles. Source water was categorized (see supplement available at stacks.iop.org/ERL/7/035301/mmedia) as (i) uncontaminated surface water, thereafter termed SURF, (ii) uncontaminated subsurface water called SUB or (iii) water stemming from the subsurface oil plume, labeled PLUME (table 1). PLUME water was identified by an elevated fluorescence signal, an oxygen deficit or a transmissometer signal indicative of the hydrocarbon plume (see supplement available at stacks.iop.org/ERL/7/035301/mmedia).
Table 1. Roller tank experiments on marine snow formation. SURF = uncontaminated surface water, sterile SURF was pre-filtered and autoclaved, SUB = uncontaminated subsurface water, PLUME = subsurface water with a signature consistent with the presence of oil. GoM-oil was collected in May 2010 from the surface; LA-oil: EPA-API, reference oil, Louisiana crude oil WP681, mix = samples were pooled together from different casts (see text for details).
| Sample collection | Lat (°N) | Long (°W) | Source water | Addition | % oil added (v/v) | N# | Treatment name |
|---|---|---|---|---|---|---|---|
| Exp. I RV Pelican 05–08 May 2010 | 28.74 | 88.00 | SURFa | — | — | 2 | SURF I |
| SURFa | GoM-oil | 1 | 2 | SURF I + GoM-oil | |||
| Sterile SURFa | GoM-oil | 1 | 1 | SURF I control + GoM-oil | |||
| Exp. II RV Walton Smith30 May–05 June 2010 | 28.74 | 88.43 | SUB-mix 900–1110 m SUB-mix 900–1110 m | — | — | 1 | SUB II |
| 28.85 | 88.49 | GoM-oil | 1 | 1 | SUB II + GoM-oil | ||
| 28.70 | 88.56 | ||||||
| 28.72 | 88.39 | PLUME-mix 1000–1240 m | — | — | 2 | PLUME II | |
| 28.69 | 88.43 | ||||||
| 28.73 | 88.38 | ||||||
| Exp. III RV Oceanus24 Aug.–8 Sep. 2010 | 26.92 | 90.42b | SUB 450 m | — | — | 2 | SUB III-1 |
| PLUME 1140 m | — | — | 2 | PLUME III-1 | |||
| 28.85 | 88.68c | SUB 40 m | — | — | 2 | SUB III-2 | |
| SUB 550 m | — | — | 2 | SUB III-3 | |||
| PLUME 133m | — | — | 2 | PLUME III-2 | |||
| PLUME 310 m | — | — | 2 | PLUME III-3 | |||
| 28.80 | 88.07d | PLUME 1300 m | — | — | 1 | PLUME III-4 | |
| PLUME 1300 m | LA-oil | 0.06 | 1 | PLUME III + LA-oil | |||
| PLUME 1300 m | LA-oil | 0.06 | 1 | PLUME III + LA-oil+ beadse | |||
| 27.50 | 87.18f | SURF + Trichodesmium | — | — | 2 | SURF III-1 | |
| SURF + Trichodesmium | LA-oil | 0.06 | 2 | SURF III + LA-oil-1 | |||
| 27.50 | 88.20g | SURF + Trichodesmium | — | — | 2 | SURF III-2 | |
| SURF + Trichodesmium | LA-oil | 0.06 | 1 | SURF III + LA-oil-2 |
aSome results of these experiments are also published in Ziervogel et al (2012), where SURF I= SW, SURF I+ GoM-oil= SW+ oil, SURF I control+ GoM-oil= control SW+ oil. bRV Oceanus station 9.01. cRV Oceanus station 18.01. dRV Oceanus station 16.10. eInert particles (1.025 μm ± 0.01 μm; 106 l−1 final conc.) fRV Oceanus station 2. gRV Oceanus station 3.
Whereas collection sites were in the direct vicinity of the well head during the two first cruises, sites farther to the south and to the east, towards DeSoto Canyon, were also included during the cruise in August (figure 1, tables 1 and 2) because of anecdotal reports of additional plumes in these areas. Uncontaminated surface water was collected well outside of the contaminated area. Whereas in May–June PLUME water was identifiable by a clear fluorescence signal, these identifying parameters were much less distinct by late August (figure S1 available at stacks.iop.org/ERL/7/035301/mmedia). Subsurface turbidity signals indicated a (past) plume region, but designations of plume III (plume samples in experiment III) should be interpreted with caution. Subsurface (SUB) water was collected below the surface at depths that showed no indication of the plume signal (for more detailed information on the characteristics of source water and its collection see supplement and supplement figure S1 available at stacks.iop.org/ERL/7/035301/mmedia). None of the source waters used in these experiments contained marine snow.
Table 2. Qualitative observations of marine snow formation in experiments.
| Exp. | Treatment name | Aggregates: # of aggregates ∗ diameter (time of first appearance) | Marine snow formation mechanism | Sinking behavior |
|---|---|---|---|---|
| I | SURF I | No (≤504 h)a | — | — |
| SURF I + GoM-oil | Yes: 25*5 mm → 1*30 mm (24–168 h) | Mucus webs & flocs | Rapid sinking | |
| SURF I control + GoM-oil | Yes: 1*20–30 mm (168–240 h)b | Mucus webs | Rapid sinkingb | |
| II | SUB II | No (≤672 h) | — | — |
| SUB II + GoM-oil | Yes: 25*5 mm (24 h) | Flocs | Rapid sinking | |
| PLUME II | No (≤672 h) | — | — | |
| III | SUB III-1 | No (≤48 h) | — | — |
| PLUME III-1 | No (≤48 h) | — | — | |
| SUB III-2 | No (≤144 h) | — | — | |
| SUB III-3 | Yes: 1*0.5 mm (144 h) | ? | — | |
| PLUME III-2 | Yes: 5*0.5 mm (144 h) | Flocs or OMAs | — | |
| PLUME III-3 | Yes:1*10 mm (144 h) | Flocs or OMAs | Float | |
| PLUME III-4 | No (≤216 h) | — | — | |
| PLUME III + LA-oil | No (≤216 h) | — | — | |
| PLUME III +LA-oil + beads | No (≤216 h) | — | — | |
| SURF III-1 | Yes: 1*20–50 mm (20–96 h) | Phytoplankton aggregates | Float, >70 h sinking | |
| SURF III + LA-oil-1 | Yes: 4*1–5 mm (20–96 h) | Phytopl.-oil aggregates | Float, >70 h float | |
| SURF III-2 | Yes: 5*1–10 mm (28–92 h) | Phytoplankton aggregates | Float | |
| SURF III + LA-oil-2 | Yes: 5*1–10 mm (28–92 h) | Phytopl.-oil aggregates | Float |
aOne highly transparent aggregate in the tank formed after 72 h. bThe large aggregate observed at the end of the incubation was very similar to those of SURF I+ GoM-oil, but formed from mucous strands anchored to the oil layer at the surface. Phytopl.-oil aggregates= phytoplankton aggregates that contained visible oil droplets.
Experiments to investigate the effect of oil addition on the formation of aggregates required the addition of oil to some of the source seawater samples. Oil-rich surface water was collected less than 1 nautical mile from the spill site using a bucket from the RV Pelican (5 May–8 May 2010) and was designated as GoM-oil (see also Ziervogel et al 2012). Alternatively, Louisiana light crude oil (EPA-API, Reference oil WP681, from Fisher), termed LA-oil was also used to amend source water. Whereas the LA-oil was pristine, sterile and similar in type to the oil released during the Deepwater Horizon spill, the GoM-oil was a seawater–oil solution that had undergone various changes due to fractionation of oil components during upward transit, physical–chemical weathering and microbial activity. For example, most or all of the alkanes below C-15 were stripped from the original source oil in the weathered oil found at the surface (Federal Interagency Solution group: Oil Budget Calculator Science and Engineering team 2010). GoM-oil also included a microbial community able to utilize oil components (Ziervogel et al 2012).
We investigated the aggregation potential of uncontaminated surface or subsurface water in the presence or absence of GoM-oil or LA-oil, as well as the aggregation potential of plume water from different locations (table 1). Uncontaminated source water without additions was used as controls. All experiments were conducted on roller tables, which keep particles, including aggregates, in suspension, rather than allowing them to settle onto container walls. When bottles are rotated on roller tables, turbulence develops (Jackson 1994) mimicking conditions near the surface. When rolling tanks (cylinders) are filled free of bubbles, solid body rotation is established, mimicking a quiet water environment (Ploug et al 2010). Both types of containers promote aggregation, and bottles were used in experiments I and II and rolling tanks were used in experiment III. All experiments were conducted in the dark.
2.2.1. Marine snow formation experiment I.
After collection SURF I samples were stored in 500 ml glass jars with a headspace and transported back to the laboratory at the University of North Carolina (UNC) where they were stored for four weeks at 4 °C in the dark until the onset of the experiment. The relatively long storage time was the result of time and logistical constraints. Replicate 1-L Pyrex© glass bottles (total volume 1150 ml) were filled to the 1-L mark with uncontaminated surface water and 12 ml of GoM-oil each (SURF I + GoM-oil; table 1). A control for aggregate formation was run with uncontaminated surface water only (SURF I). A second control filled with 0.1 μm filtered and autoclaved uncontaminated surface water and 12 ml GoM-oil (SURF I control+ GoM-oil; table 1) lacked the microbial community present in SURF, but did include the microbial community associated with GoM-oil. All bottles were incubated for 21 days at in situ water temperature (25 °C) at a rotation speed of 3.5 rpm. Every 1–5 days bottles were removed from the roller table and placed up-right to photographically document marine snow formation. For more details see Ziervogel et al (2012).
2.2.2. Marine snow formation experiment II.
Fourteen samples (300–400 ml each) of subsurface water collected in May/June 2010 by RV Walton Smith were transported in 500 ml glass jars to UNC where they were stored for four weeks at 4 °C in the dark. After storage samples from different casts were combined to create PLUME II, that had an elevated fluorescence signal and SUB II that showed only background fluorescence (figure S1 available at stacks.iop.org/ERL/7/035301/mmedia; table 1). Pooling of samples was necessary to obtain adequate sample volumes. Duplicate 1-L Pyrex© glass bottles (total volume 1150 ml) were filled to the 1-L mark with SUB II or PLUME II. Twelve ml GoM-oil were added to one of the SUB II bottles (SUB II+ GoM-oil; table 1). Experiments were conducted for 28 days at 4 °C (in situ temperature) at a rotation speed of 3.5 rpm.
2.2.3. Marine snow formation experiment III.
This series of experiments was conducted onboard RV Oceanus between 24 August and 5 September 2010 using surface, subsurface and plume water collected at five different sites (figure 1, table 1). The aggregation potential of uncontaminated subsurface water collected from between 450 and 550 m (SUB III-1,-3) as well as from the chlorophyll maximum (SUB III-2) was compared with that of PLUME water (PLUME III-1 to -4) collected at three sites and four depths (table 1). The potential of source water to form marine snow in the presence of pristine oil (LA-oil) was tested at three sites (SURF+ LA-oil-1, -2 and PLUME+ LA-oil; table 1). In August, Trichodesmium sp., a colony forming autotrophic cyanobacterium, was abundant in surface waters. Because particle concentrations and size appreciably impact aggregation rate we ensured that concentration of colonies in each sample was identical. Thus fifty hand picked Trichodesmium sp. colonies, collected with a 64 μm hand net, were placed into 0.2 μm filtered surface water for SURF III experiments. Inert particles, beads (table 1) were added to a replicate of the PLUME water with LA-oil, to increase particle numbers and thus encourage coagulation (PLUME III+ LA-oil+ beads). All experiments were conducted shortly after sampling in rolling tanks (total volume: 1.7 L, rotation speed of 2.4 rpm) that were filled bubble free. Incubations were conducted at 28–30 °C and marine snow formation was documented visually every 1–2 days and marine snow investigated microscopically upon sampling.
3. Results and discussion
3.1. Characteristics and sinking behavior of in situ collected marine snow
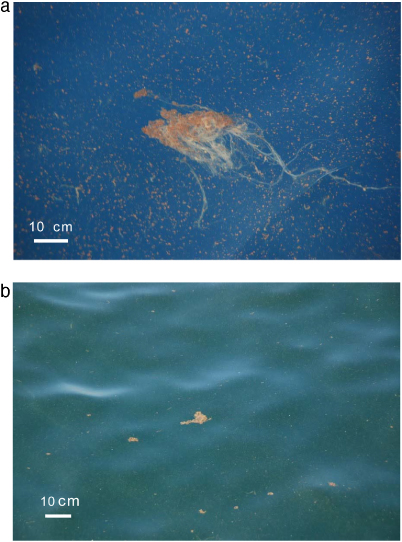
The large, mucus-rich marine snow particles observed in May in situ differed both in size and in their consistency from marine snow usually observed in the GoM or elsewhere. This GoM-snow existed in a size continuum, including small, more compact marine snow particles as well as those several centimeter in size. Stringy, larger marine snow (<10 cm) with veil-like mucous threads that created a web-like structure was also common (figure 2).
Figure 2. Mucus-rich marine snow observed in situ beginning of May 2010 in the GoM in the vicinity of oil layers at the surface. (A) Stringy and (B) fluffy types of marine snow were observed. Scale bars are 10 cm.
Download figure:
Standard imageUnfortunately, no systematic documentation exists on the in situ sinking velocity of the marine snow observed in the GoM in May 2010, but casual observations suggest that marine snow remained suspended at the surface. Although we acknowledge the probable impact of collection, transport and storage of these aggregates, we did observe a similar behavior of the collected marine snow within the vials, as roughly half of the collected GoM-snow still floated at the surface of the vials after one month of storage. In April 2012, two years later, some individual marine snow particles within the vials which were kept dark and cool were still positively buoyant, although most material had sunk. Aggregates were considered positively buoyant if they moved upwards in the water of the vial when disturbed. In May 2010, one month after collection some of this positively buoyant GoM-snow was small (1–5 mm), but larger GoM-snow (>10 mm) was also observed floating. Some of the smaller floating GoM-snow seemed caught in the oil layer at the surface, forming a web-like structure. The non-buoyant GoM-snow that had sunk to the bottom of the vial within the first month was small (≤5 mm), but also had a very fluffy appearance. Sinking velocity and density were determined on this settled GoM-snow but no appropriate method could be conceived for making sinking velocity measurements on buoyant GoM-snow. All vials had an oily rim, with most having a clearly visible oil layer at the surface.
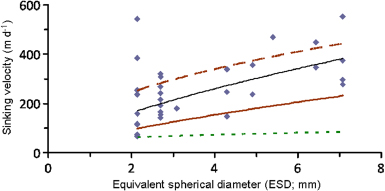
The sinking velocity of collected, settling GoM-snow ranged over one order of magnitude from 68 to 553 m d−1, comparable to that of natural marine snow in the GoM (Diercks and Asper 1997). The sinking velocity of this GoM-snow was loosely size dependent (figure 3) and bracketed by that of other types of marine snow (Alldredge and Gotschalk 1988, Ploug et al 2008), implying the potential for rapid sedimentation.
Figure 3. Sinking velocities versus equivalent spherical diameter (ESD) of 32 individual, sinking marine snow particles collected from the oily surface layer in the GoM (GoM-snow) followed the relationship w = 101 ESD0.68 (black line) but scatter was very high (r2 = 0.32). For comparison the relationship of sinking velocity versus ESD of natural marine snow measured in situ off California (w = 50 ESD0.26; dotted green line, Alldredge and Gotschalk 1988) and of laboratory made diatom (solid brown) and coccolithophore (hatched brown) aggregates measured in vitro (w = 56 ESD0.72 and 176 ESD0.47; Iversen and Ploug 2010) are also shown.
Download figure:
Standard imageCalculated excess densities (compared to seawater) of GoM-snow was also a function of size (figure 4(A)) and ∼60% of the GoM-snow fell between the excess density of diatom and coccolithophores aggregates (see Iversen and Ploug 2010). But the variability in their excess density was large (0.001 ± 0.001 g cm−3), with values smaller and larger than similarly sized diatom- or coccolithophore aggregates. This suggests either that our collected GoM-snow was very heterogeneous with respect to their composition or that the GoM-snow we collected represented different time points in an aging process. Oil droplets were clearly visible within some of the GoM-snow. The inclusion of oil components will significantly impact the excess density of marine snow and additionally make excess density liable to change over time as the oil ages and weathers. Incremental weathering of oil components within the GoM-snow would be an obvious explanation for the large variability in their characteristics.
Figure 4. (A) Excess density of GoM-snow collected from the oily surface layer in the GoM versus their respective ESD, calculated from sinking velocity and size. Lines represent similarly calculated excess densities (Iversen and Ploug 2010) of laboratory made aggregates consisting of diatoms (solid) and coccolithophores (hatched). (B) Frequency distribution of the excess densities of the solid component particles making up GoM-snow. Symbols of particles stemming from the same marine snow particle are identical.
Download figure:
Standard imageWhen determining the solid hydrated density of GoM-snow constituents, the GoM-snow disintegrated into its components. The excess densities of these individual component particles ranged widely between 0.07 and 0.36 g cm−3, but the averages of all component particles of each measured GoM-snow were similar (0.392 ± 0.011 g cm−3). This supports the idea that the heterogeneity in composition within each aggregate was high, whereas differences between GoM-snow were small. Components with an excess density of 0.20–0.24 g cm−3 were most abundant in all measured GoM-snow (figure 4(B)). This is a very high excess density, above that of the constituents from diatom aggregates, which are ballasted by opal frustules. Excess densities of the components of laboratory made diatom aggregates ranged between 0.13 and 0.19 g cm−3 (Gaerdes et al 2011).
By comparing the average excess density of the solid portion of the GoM-snow with the average excess density of the whole GoM-snow aggregate, we estimated the average porosity (percentage of volume occupied by water as opposed to solid particles) of our GoM-snow to be 99.7%. Porosity of natural aggregates ranges from 97% to 99.9% (Alldredge and Gotschalk 1988), and laboratory made aggregates may have a porosity as low as 96% (Ploug et al 2008). It is possible that when determining the excess porosity of the component particles, substances with a density lighter than seawater were missed. This would correct the porosity estimates downward, possibly to 99.4% or lower. Such porosity calculations ignore the presence of mucus and it has been estimated that TEP, for example, contribute 5–10% to diatom aggregates. Visual observations suggest that the contribution of mucus was, at least originally very high in the GoM-snow. However, TEP are positively buoyant (Azetsu-Scott and Passow 2004), and our density and sinking velocity measurements suggest that the contribution of mucus must have declined by the time the GoM-snow sank. Overall, one month after their formation a large fraction of the GoM-snow exhibited characteristics similar to other types of marine snow.
3.2. Mechanisms of marine snow formation
Based on the roller tank observations we suggest three formation mechanisms for marine snow in the GoM after the spill. The first mechanism involves the formation of marine snow from mucus threads associated with the surface oil layer. This mechanism was most obvious in the treatment where GoM-oil was added to uncontaminated, sterile surface water (SURF I control +GoM-oil; table 2). Mucous strands developed from the oil layer at the surface, and formed a web of strings hanging into the water (figures 5(D) and (E)). After ∼7 days, this stringy mucus web coalesced and formed a very large marine snow particle. The initial step of this formation mechanism resembles the formation of biofilms, where a mucus layer is formed by a microbial community. The interface between the GoM-oil, the water and the atmosphere may have provided the 'surface' for the formation of such 'floating biofilms'. Thus the formation of such mucus webs is most likely restricted to the ocean surface. The responsible microbial community must have been present in the GoM-oil, as the source water was sterilized. Similar mucus webs also formed in the treatment with untreated source water amended with GoM-oil (SURF I+ GoM-oil; table 2), where, at day 21, flocs (see second formation mechanism below) were trapped by the mucus web of strings that had developed at the base of the oil layer floating at the surface (figures 5(D) and (E); see also figure 1 in Ziervogel et al 2012). A similar mucus web did not form one month later in the treatment where GoM-oil was added to subsurface water (SUB II+ GoM-oil; table 2) possibly because of a decreased metabolism of warm-water adapted, oil-degrading bacteria at an incubation temperature of 4 °C compared to 25 °C.
Figure 5. (A) Marine snow formation in SURF I +GoM-oil. (B) About 25 flocs (5 mm diameter) formed within 24 h of the roller table incubation. Similar flocs also formed in SUB II+ GoM-oil. (C) After seven days flocs coalesced to form one very large 30 mm marine snow particle per bottle. The marine snow had oil droplets incorporated (white arrows). (D) Additionally, at day 10, mucous webs, which were anchored at the base of the floating oil layer became visible. This formation of a mucus web was also observed in SURF I control+ GoM-oil. (E) These mucus webs were very sticky and flocs stayed attached to these mucus strands upon collision. Scale bars in (B)–(D) are 5 mm.
Download figure:
Standard imageWhereas the details of the interaction between the microbial communities harbored in the GoM-oil and the oil components await further discoveries, our results strongly suggest an active participation of bacteria during the formation of mucus webs. Oil-degrading bacteria, which were abundant in GOM-oil (Yang et al 2012), presumably produced ubiquitous amounts of EPS to emulsify crude oil (Hino et al 1997), while utilizing some of the components of the oil (Ziervogel et al 2012). Eventually these mucus–oil webs collapsed upon themselves, similar to old spider webs, thereby forming mucus-rich marine snow. This marine snow would be extremely sticky and easily scavenges all types of other particles present in the water, therewith providing nuclei for the formation of very large mucus-rich marine snow. The ability of mucus web-derived marine snow to scavenge and trap other particles and subsequently grow in size was demonstrated in our experiment with GoM-oil and untreated surface water (SURF I+ GoM-oil), where flocs were scavenged by the mucus web (figure 5(E)). Although formed in the absence of oil by different mechanisms, mucilage-rich marine snow in the Adriatic Sea also effectively scavenges other particles when sinking (Del Negro et al 2005, Flander-Putrle and Malej 2008).
A second mechanism involves the direct formation of aggregates due to collision and sticking of particles and was observed in parallel to mucus web formation in SURF I +GoM-oil. The marine snow formed via this mechanism was more roundish and very fluffy and will be called flocs. About 25 flocs of ∼5 mm in diameter formed in the 1 l containers within 1 day (figure 5(B)). These flocs were eventually trapped by and incorporated into the mucus web-derived marine snow that formed simultaneously at the base of the oil layer (see above, figure 5). Similar flocs also formed when GoM-oil was added to uncontaminated subsurface water in July (SUB II+ GoM-oil; figure 6, table 2). We suggest that the formation of these flocs involved the production of particles due to interactions between specific oil components, bacteria and natural suspended matter because particle concentrations in the source waters were too low to support the formation of such large amounts of marine snow via coagulation. In fact unaltered surface water (SURF I) did not form flocs although one transparent aggregate formed (Ziervogel et al 2012). Thus the formation of large flocs in un-enriched but oil-contaminated seawater suggests increased formation of particulate material in the presence of oil. We hypothesize that the aggregation of polar components of oil were one of the main driving forces for the formation of flocs. Polar crude oil components, such as asphaltenes and resins, are known to form oily particulate matter (i.e. water-in-oil emulsions) in oil-contaminated surface waters (Patin 1999). Reddy and co-authors (Reddy et al 2011) reported that polar hydrocarbons made up 10% of the oil fraction in one of their samples taken from the damaged riser pipe in late June 2010. These authors also point out that many of these polar components are resistant against biodegradation and weathering which would possibly lead to an accumulation of polar hydrocarbons over time. The abiotic formation of macro-gels like TEP from EPS released by bacteria in response to the presence of oil and any suspended particles may have further contributed to the formation of particulate matter incorporated in these flocs.
Figure 6. SUB II +GoM-oil. Flocs in experiments with subsurface water and GoM-oil formed within 24 hrs and were similar in size and appearance to the ones in figures 5(A) and (B) (scale bar is 5 mm).
Download figure:
Standard imageA third mechanism of marine snow formation was observed in the presence of the cyanobacterium Trichodesmium (SURF III; table 2), which formed aggregates via coagulation of the colonies. Trichodesmium commonly forms dense blooms in the Gulf of Mexico (Holl et al 2007), and these coagulate readily in the absence of oil, but in the presence of oil, oil droplets were incorporated in the aggregates (SURF III+ LA-oil). In our experiments the Trichodesmium aggregates initially differed in appearance from the GoM-oil snow observed in situ. The former were bright green and very fragile, clearly consisting of the cyanobacteria. Clearly visible oil drops were incorporated into these aggregates in treatments with LA-oil. Aggregates with oil turned white, mucus-rich and stringy within hours, much more rapidly than those without oil. Microscopic investigation of aggregates that included oil revealed abundant phyto-detritus rather than the tuffs or puffs typical for Trichodesmium. More rapid degradation of algae in the presence of oil was also suggested by measurements of the pigment signature and Fv/Fm by Advanced Laser Fluorometer, ALF (Chekalyuk and Hafez 2008), which confirmed that after >28 h the photosynthetic apparatus of these algae was totally dysfunctional in treatments with oil additions. In contrast it was still functional, although inactive in treatments without oil (Chekalyuk 2012). The concentration of TEP in Trichodesmium aggregates seemed higher in the presence of oil but possibly this is a methodological artifact, as oil interferes with colorimetric methods. Thus the data are not shown.
In our experiments, the addition of LA-oil did not appear to cause or increase the formation of marine snow, although this oil was incorporated into phytoplankton aggregates and impacted their aging. Our results thus suggest that the impact of the unaltered LA-oil on marine snow formation differed appreciably from that of the weathered water–oil mix of the GoM-oil, but we have no direct comparison as source water and thus microbial community also differed between experiments with LA-oil and those with GoM-oil (tables 1 and 2). However, we suggest that the LA-crude oil lacked components derived from chemical and biological weathering important to initiate the biological production of EPS or to promote the formation of the oil particles that formed flocs.
Marine snow formed in the laboratory by all three mechanisms (table 2) resembled the GoM-snow observed in situ (figure 3). Stringy mucous-rich webs, fluffy flocs and oil-contaminated, rapidly degrading Trichodesmium aggregates were similar in size and appearance to the marine snow that formed in situ at the surface around the spill site. While inclusion of oil droplets in phytoplankton aggregates may trap oil in marine snow in most oil-contaminated environments, we propose that the formation of mucous webs may particularly apply to the Gulf of Mexico where natural microbial communities are likely adapted to the presence of oil and gas injected through many natural seeps even without a major oil spill. We would expect the formation of flocs due to particles formed from oil to depend largely on oil composition, suggesting that floc formation would differ depending on the history of the oil, for example the release depth.
3.3. Marine snow formation at depths
Of seven experiments conducted with water designated as PLUME water, marine snow formed in two, whereas no marine snow formed in the other five (table 2), even when LA-oil was added (two experiments). Marine snow formed only in the samples of the shallower plume water acquired near the shelf in late August (PLUME III-2, -3). The formation of marine snow in PLUME water suggests that subsurface marine snow formation can act as an important intermediate product for chemically dispersed gas and hydrocarbons at depths. The marine snow formed in PLUME III-2 and -3 may represent oil–mineral aggregates (OMAs). Numerous studies have demonstrated that elevated levels of suspended minerals in oil-contaminated waters lead to the formation of OMAs which are usually small (≤0.1 mm) but vary in size (Omotoso et al 2002, Stoffyn-Egli and Lee 2002). Different types of OMAs exist, depending on mineral and oil type and on environmental conditions. High concentrations of suspended particles were indicated by the beam transmission signal at PLUME III-2 and -3 (figure S1 available at stacks.iop.org/ERL/7/035301/mmedia). The presence of small OMAs may have led to the formation of larger aggregates in our incubations. Although most OMAs sink, some may float (Stoffyn-Egli and Lee 2002), as did the marine snow in our experiment (table 2).
The absence of marine snow formation in the PLUME water that had a clear fluorescence signal (PLUME II, figure S1 available at stacks.iop.org/ERL/7/035301/mmedia) indicates that the presence of dissolved gas and hydrocarbons alone does not suffice to trigger marine formation (table 2). Possibly the absence of sticky particulate matter of either mineral or bacterial origin may explain the lack of aggregate formation. Floc formation due to particle formation from oil and EPS could potentially occur at any depth, given the 'correct' oil and microbial community. EPS production of bacteria is, however, species-specific (Myklestad 1995), and microbial communities in GoM-oil were different from those in the plume in late May (Yang et al 2012). It is therefore likely that the microbial community inside the plume at the time of our sampling produced less EPS compared to those associated with the GoM-oil. PLUME III samples had a weak or no fluorescence signal, suggesting that much of the oil had been diluted, dissipated, incorporated or otherwise utilized by August, although a signal in beam transmission marked these anomalous layers (figure S1 available at stacks.iop.org/ERL/7/035301/mmedia). However, oil concentrations may very well have been too low to initiate floc formation in plume water in August. The lack of marine snow formation in the treatment where artificial particles were added to plume water (PLUME III +LA-oil +beads; table 2) further emphasizes the importance of stickiness for the formation of marine snow. It is currently unclear if marine snow formed in situ in the subsurface plumes, and given the possible different levels of dissolved oil and gas in our samples, our interpretation of the results from experiments with PLUME water are challenging. Our laboratory results demonstrate the importance of the quality and quantity of suspended matter, oil components and the microbial community for the formation of flocs, OMAs or other types of aggregates at depth.
4. Conclusions
Based on our experimental results we hypothesize that three mechanisms may have lead to the high concentration of marine snow observed near the surface in the GoM after the spill: (1) production of mucous webs through activities of bacterial oil-degraders associated with the floating oil layer at the surface, (2) the coagulation of oily particulate matter produced through interactions of oil components with suspended matter including macro-gels and (3) the coagulation of phytoplankton with oil droplets incorporated into aggregates. High concentrations of minerals may have contributed to the formation of OMAs at depths, especially near the shelf.
Characteristics of marine snow collected in the GoM after the spill and our experimental results suggest that the sinking behavior of oil-based marine snow follows a temporal progression: in situ marine snow formed at the surface initially remained floating. Most likely buoyancy was attained from a high content of oil components and mucus with a low density. While the marine snow in the GoM disappeared within a month of its initial appearance, presumably mostly due to settling, the collected marine snow (stored dark at 4 °C) had only partially sunk with some still floating after a month and even after two years. Cold, dark storage of the collected snow may have retarded its further aging compared to that of marine snow in situ. The measured sinking velocity of the non-floating snow was within the range expected for phytoplankton or miscellaneous aggregates of that size. Measurements of hydrated porosity of the component particles of the collected oil-rich marine snow exhibited a very large range, consistent with the idea that this oil-rich marine snow was made up of a wide variety of components characterized by different densities. Experimentally generated snow also floated or sank. In the absence of oil, floating marine snow is the exception; only fresh aggregates from cyanobacteria float consistently, but sink as they age. We suggest that as oil components that are trapped in aggregates age due to weathering or microbial activity their excess density and thus sinking velocity increases.
The formation and sinking of oil-derived marine snow in surface as well as subsurface waters is a possible pathway that determines the distribution and cycling of oil within the ecosystem. Sinking oil-derived aggregates would contain fossil carbon within mucus and bacteria biomass and presumably also unassimilated hydrocarbons. As described for natural marine snow (Steinberg 1995, Green and Dagg 1997, Dilling et al 2004), sinking marine snow with incorporated oil becomes an important food source for pelagic zooplankton, fish as well as benthic organisms. Currently it is unknown how the fossil carbon entered the food web after the Deepwater Horizon spill (Graham et al 2010), however, planktonic grazing on oil-derived marine snow could represent an efficient pathway for this carbon to enter higher trophic levels. Another fraction of sinking marine snow will reach the sediments, potentially impacting benthic communities but the effects depend on wave action, currents, sediment type and benthic communities (Lee 2002, Niu et al 2011).
Acknowledgments
Special thanks for funding through a RAPID by NSF (OCE-1045330) to UP, VA and AD Funding for KZ came from an NSF RAPID (OCE-1045115) awarded to Andreas Teske (UNC). This work was also partially supported by the research consortium ECOGIG (GoMRI I). We thank the crews from all three cruises; this work could not have been done without them, as well as J Montoya, chief scientist on RV Oceanus. We also thank Luke McKay, Tingting Yang and Lisa Nigro (all UNC) for collecting the samples aboard ship for roller tank experiments I and II. Last but not least we thank our two referees for their very constructive, positive and speedy reviews.