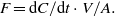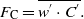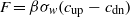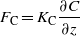Abstract
This letter provides an overview of the available measurement techniques for nitrous oxide (N2O) flux measurement. It is presented to aid the choice of the most appropriate methods for different situations. Nitrous oxide is a very potent greenhouse gas; the effect of 1 kg of N2O is estimated to be equivalent to 300 kg of CO2. Emissions of N2O from the soil have a larger uncertainty compared to other greenhouse gases. Important reasons for this are low atmospheric concentration levels and enormous spatial and temporal variability. Traditionally such small increases are measured by chambers and analyzed by gas chromatography. Spatial and temporal resolution is poor, but costs are low. To detect emissions at the field scale and high temporal resolution, differences at tens of ppt levels need to be resolved. Reliable instruments are now available to measure N2O by a range of micrometeorological methods, but at high financial cost. Although chambers are effective in identifying processes and treatment effects and mitigation, the future lies with the more versatile high frequency and high sensitivity sensors.

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Almost 90% of global N2O emissions are a result of the microbial processes nitrification and denitrification (e.g. Wrage et al 2001) in soils and waters. Emission and production rates are governed by external drivers, principally nitrogen availability, redox potential and temperature (Skiba and Smith 2000). Consequently the agricultural sector, with its high usage of nitrogen, is globally the largest anthropogenic source of N2O (figure 1). Changes in land use, for example switching from a forest to arable or grassland also affect N2O emissions; and natural N2O emitters, such as forests, aquifers, rivers and estuaries are enhanced by nitrogen leaking and deposition mainly from diffuse agricultural sources (Reay et al 2012, Skiba et al 2012).
Figure 1. The global anthropogenic N2O budget, grouped by emission sectors and the continents. Data are from the Emission Database for Global Atmospheric Research (EDGAR) for year 2008 European Commission, Joint Research Centre (JRC)/PBL Netherlands Environmental Assessment Agency. 'Energy' includes mobile and stationary fuel combustion, fossil fuel fires, aviation and navigation. Note that the 'Biomass burning' sector for Africa is larger than the agricultural sector. 'Industry' emissions are mainly from adipic and nitric acid production plants. 'Agriculture' includes categories: manure management, manure in pastures, ranges and paddocks, direct soil emissions, diffuse emissions and atmospheric N deposition of NO and NH3. 'Waste' includes solid waste, waste water handling, incineration, other waste.
Download figure:
Standard image High-resolution imageEmission rates are typically very variable both in space and time (e.g. Zhu et al 2013). Small scale heterogeneity of physical and chemical soil properties, seasonality (rainfall and temperature) and agricultural management (e.g. fertilization, plowing, irrigation) influence microbial production. In particular grazed fields with urine and dung delivering patches of concentrated nitrogen show large spatial variability in emissions throughout the year (Lesschen et al 2011). The temporal structure of the emission pattern is further complicated by fertilizer spreading, the method of spreading and the type of fertilizer used. Significant peaks in the emission can typically occur between 0 and about 21 days after spreading mineral nitrogen, often triggered by rain (Skiba et al 2013). For organic compounds (manures, slurries) peaks occur later and are longer lasting as microbial decomposition must precede nitrification and denitrification (e.g. Jones et al 2007).
This emission 'scheme', in combination with the measurement methods available, leads to uncertain emission estimates for the measured field. Extra uncertainty is added in the process of upscaling to the national level. Upscaling has to rely on the representativity of a limited set of measured fields for other similar fields together with often insufficient knowledge of the key driving variables that determine N2O emission (e.g. soil texture, drainage, bulk density, carbon content, pH, nitrogen availability, temperature, rainfall). Such data is scarce, even in countries were national emission inventories are supported by flux data for a wide range of environments and well documented farm and environmental records. For Europe (EU27) uncertainties of agricultural N2O emissions were estimated at around 80% (Freibauer 2008) and for a very well documented country as the Netherlands, the contribution of N2O from fertilized agricultural fields to the total GHG balance still has an uncertainty in the order of 50% (Maas et al 2009).
The principal N2O measurement method is the static chamber and has been used to measure N2O for almost 40 years. (e.g. Delwiche and Rolston 1976, Matthias et al 1978). It is the cheapest most versatile N2O measurement method, but is not able to provide the high time and spatial resolution required to improve our greenhouse gas budgets and policy making. Technological advancements of principally the laser technology, now enables high temporal and spatial resolution measurements, mainly using micrometeorological methods, at almost affordable prices. The aim of this review is to describe the main chamber and micrometeorological methods used to measure N2O, identify their pros and cons and make recommendations on appropriate use.
2. Methods for measuring N2O emissions
The first step to obtain N2O emissions is to measure N2O concentrations above atmospheric levels of approximately 0.32 μl l−1. With these data available N2O flux rates can be derived using several different flux methods. Sensors and methodologies are introduced below.
2.1. Concentration measurements
2.1.1. Gas chromatography.
The principle of gas chromatography is the separation of a compound into its molecular constituents. For N2O the sample is injected through a sample loop (typically 0.25–1 ml) into a carrier gas stream of N2 (or He) of a gas chromatograph (GC) fitted with an electron capture detector (Wang et al 2010, Kelliher et al 2013). The volume of sample injected should be at least two times larger than the size of the sample loop. Analysis takes 2–10 min and in near background conditions (around 310 μl l−1) an accuracy of 0.2 μl l−1 can be obtained (e.g. Jones et al 2011). Regular (every 30 min) calibration of the system is required to enable corrections for system drift (figure 2) (Loftfield et al 1997, Smith and Conen 2004). Accuracy of GC measurements varies between laboratories, instruments and operators (Venterea et al 2009, Kelliher et al 2013). We would recommend that each operators perform regular reproducibility tests to refine and establish accuracy. It is also necessary to test all GC detectors for possible contamination with O2 (Parkin and Venterea 2010) or CO2 (Zheng et al 2008).
Figure 2. The left picture is an example of a typical GC fitted with an electron capture detector for N2O analysis and a flame ionization detector for CH4 and CO2 analysis (left) linked to an autosampler containing the sample vials. Gas samples are usually collected by syringe and stored in either pre-evacuated vials or, as shown in the right picture, by flushing a large sample volume through a small vial. As N2O is a stable gas vials can be stored for several weeks before analysis.
Download figure:
Standard image High-resolution image2.1.2. Infrared techniques.
Infrared detection (IR) techniques exploit the ability of gases (e.g. H2O, CO2, CH4, N2O and NH3) to absorb infrared light at unique wavelengths. Sample gas is either pumped into a measurement cell where the IR beam is illuminating the sample (closed path system) or the IR beam can be used in the outside air (open path system). To date, only closed path measurement systems are used for N2O, although open path systems are currently under development. Commonly used IR detectors are (1) Fourier transform infrared spectrometers (FTIR), which use a broadband thermal system to scan though the IR spectrum, and thereby measure a whole suit of gases simultaneously (Galle et al 1994, Schäfer et al 2011); (2) photo-acoustic instruments, combining opto-acoustics with a broadband IR source (Iqbal et al 2013); (3) laser-based systems tuned to the unique absorption line of a specific trace gas, such as tunable diode laser (TDL) (Mammarella et al 2010) or quantum cascade laser (QCL) spectrometers and cavity ring-down systems (CRD) (Kroon et al 2007, 2010) (figure 3).
Figure 3. Infrared N2O sensors are robust field instruments. This Quantum Cascade Laser spectrometer for CH4, N2O and NH3 (Aerodyne Instruments, Massachusetts) is deployed in an intensively managed grasslands (NL).
Download figure:
Standard image High-resolution image2.1.3. Pros and cons of GC and IR systems.
Gas chromatographs used for soil N2O flux measurements are easy to use and affordable laboratory instruments. They require a continuous supply of high purity carrier gases and have a much poorer detection limit than most IR systems. Gas samples can be collected in small vials and shipped abroad for analysis, if locally GC are not available (e.g. Hergoualc'h et al 2008).
Infrared systems are considerably more expensive than GCs and require experienced maintenance. However, they perform measurements at frequency up to 20 Hz at a sensitivity >500 times better than by GC. For example the CEH GC autochamber system has a detection limit of 0.2 μl l−1 for N2O compared to a detection limit of 0.001 μl l−1 of the tunable diode laser (Jones et al 2011) and of 0.000 03 μl l−1 for a 2010 Aerodyne quantum cascade laser (Famulari 2013).
2.2. Flux measurement methods
2.2.1. Chamber methods.
Chamber measurements have the main advantage that the concentration signal is amplified significantly so that smaller emissions can be evaluated with a given, low instrumental precision. Sensors used for chamber measurements do not need to be fast response sensors. Gas chromatographs and opto-acoustical instruments (Innova, Bruel & Kjaer, DK) are universally used to measure soil respiration rates, CH4 and N2O fluxes (e.g. Flechard et al 2005). The term 'fluxes' describes both emission and uptake; for N2O emissions tend to be more important. Manual chambers are cheap to make, do not require electricity except small batteries in some cases and are easily transported to remote areas (figure 4). Some authors suggest that it is essential to (i) mix air inside the chamber using a small fan, (ii) install a vent hole or tube to avoid effects of pressure differences between in- and outside conditions, (iii) a proper sealing to the sub-surface is required and (iv) the temperature and humidity inside the chamber should not be allowed to increase too much; so insulation or water trapping inside the enclosed chamber might be needed. Recommendations can be found in Hutchinson and Mosier (1981), Parkin and Venterea (2010), Christiansen et al (2011), Rochette (2011), Clough et al (2013).
Figure 4. Chamber measurement methods measure the increasing (or decreasing) concentration inside a chamber, preferably a number of times after closing the chamber. The photo on the left is of a low cost static manual chamber, made from commercially available drainpipe (40 cm diameter), flange and an aluminum lid and used for manual sampling of the air into glass vials for subsequent analysis by gas chromatography (figure 2). The schematic diagram on the right shows a chamber connected to a photo-acoustic analyzer, the air is re-circulated between analyzer and instrument, and the increase in N2O concentration is measured once a minute. CO2 and H2O have to be scrubbed from the gas flow in order to get useful N2O data.
Download figure:
Standard image High-resolution imageChamber fluxes (F) are calculated from the increase in concentration (dC) during chamber closure (dt) and the volume of the chamber (V) enclosing surface area (A).
Most chamber studies in the past assumed a linear increase in concentration over time, however, assuming linearity may underestimate fluxes by 20–40% (Kroon et al 2008, Kutzbach et al 2007). Calculating fluxes from several concentration measurements (3–5) during chamber closure using non-linear or best-fit-model approaches (Pedersen et al 2010, Levy et al 2011, Venterea et al 2013) would reduce some of the uncertainty.
With chambers a small area (<m2) of the ecosystem can be studied without interference from other sources. Measured flux rates can be linked to environmental variables measured at the same location and time, for example soil temperature, nitrate availability, water table depth or pH, which facilitates the development of process models. Chamber measurements do, however, have a problem covering the fast temporal and spatial inhomogeneity (figure 5) of, for example, N2O emissions from a grazed field (Velthof et al 1996, Flechard et al 2007). Chamber studies are in danger of missing main peak events after rainfall or fertilization, because the experimentalists are not in the field on a particular day and chambers can only be sealed a limited number of times per day in order not to alter the microclimate inside. Some of these issues can be resolved by using automated chamber systems, capable of flux measurements every few hours (figure 6) (e.g. Smith and Dobbie 2001, Grace et al 2013). However, spatial coverage is likely to be even smaller than for manual chambers, due to their much higher costs.
Figure 5. Spatial and temporal variability of N2O fluxes measured by eight static chambers (C1–C8) from a grazed grassland in the UK (Skiba et al 2013). The arrows denote dates of N fertilizer application (52 kg N ha−1 yr−1 in March, May, June, July, 69 kg N ha−1 yr−1 in April and 35 kg N ha−1 yr−1 in August). The onset and magnitude of fertilizer induced N2O emission peaks was different for each chamber and each fertilizer event.
Download figure:
Standard image High-resolution imageFigure 6. Two chamber methods setups used to overcome the spatial and temporal variability problems associated with soil N2O fluxes. The fast chamber method (left), using the TDL or QCL as a sensor, allows many measurements at different locations in a short period of time. On the right the automatic chamber that alternates between two positions and is connected to a GC and enables many measurements from the same locations throughout a day.
Download figure:
Standard image High-resolution imageThe combination of chambers with a fast response infrared sensor can help to significantly increase the number of measurements that can be one on a single field (Hensen 2012). The fast chamber method uses 10 Hz instruments for this application so that, in general, chambers only need to be enclosed for a few minutes. Air is re-circulated between chamber and TDL or QCL and fluxes are calculated from concentration increases recorded every second. Such high frequency records of N2O accumulation allow a much more accurate flux calculation than possible from the 3–5 points available for GC analysis. With this method, a high temporal resolution of the emission landscape can be obtained (figure 6).
2.2.2. Micrometeorological methods.
Micrometeorological methods have some advantages over enclosure methods: measurements are on a larger scale, they do not interfere with the micro-environment, and they have a very high temporal resolution. These methods integrate fluxes over large plots (>10 m2) up to regional scales. There are however disadvantages to micrometeorological methods as well: they require large, uniform surfaces, and fast response infrared sensors, which are often expensive; they are introduced below. The data capture for these methods is also constrained by the atmospheric stability, which can sometimes affect measurements during night time, for example; overall, a micrometeorological system will have better temporal coverage than any enclosure system.
2.2.2.1. Eddy covariance (EC)
The eddy covariance (EC) method is widely used since a few decades (e.g. Aubinet et al 2000, Baldocchi 2003); the emission/deposition flux (FC) of a gas is defined as the covariance between the vertical wind speed (w) and the gas concentration (C) itself measured at one point as follows:
Eddies very efficiently mix high concentration air from nearby sources with background air, and a correlation (or anti-correlation) between vertical wind and concentration indicates up- or down-ward transport of the gas (e.g. Stull 1988). To apply EC, fast sensors are required for both wind (ultra-sonic anemometers) (e.g. Kaimal and Gaynor 1991) and concentrations (see section 2.1): this means the costs for equipment and expertise are relatively high.
Eddy covariance measurements of N2O were made possible by the development of fast response laser instruments, the tunable diode lasers, which have mostly been superseded by quantum cascade laser, as these are more robust, easier to use and have higher sensitivity. Examples of measurements for N2O with TDL's are described in Jones et al (2011) or Laville et al (1997) and of QCL measurements including a discussion on uncertainties in EC measurements can be found in Kroon et al (2007, 2010). EC measurements have been mainly used to study fieldscale N2O fluxes from agricultural fields, see figures 7 and 8 (Wienhold et al 1994, Laville et al 1997, Kroon et al 2007, Neftel et al 2010), and for above canopy N2O flux measurements in forests (Shaw et al 1998, Pihlatie et al 2005).
Figure 7. Eddy covariance flux measurements at Zegveld (NL) over a manured field. Left: mast with an ultra-sonic anemometer and sample inlet line. Right: the sample line is connected to a TDL housed inside the electrically powered mobile laboratory.
Download figure:
Standard image High-resolution imageFigure 8. An example day of mixing ratios (1 ppb = 1 ng l−1) of N2O, CO2 and H2O recorded at 10 Hz by the QCL (three bottom traces), and calculated N2O fluxes (top trace). These measurements were made from a grassland in England fertilized with slurry.
Download figure:
Standard image High-resolution image2.2.2.2. The relaxed eddy accumulation (REA) method
REA is a conditional technique (Businger and Oncley 1990) that has been widely used in the past two decades for a range of gases, including N2O (e.g. Pattey et al 2006); unlike EC it allows slow response concentration analyzers. In that sense, REA is the 'cheap version' of EC: it uses the same sonic anemometer to measure the vertical wind speed w, but sampling air into updraft and downdraft reservoirs (e.g. Tedlar bags for non-reactive gases), at constant flow rate, based on the sign of w. The flux is expressed as:
where β is an empirical proportionality coefficient (generally in the range 0.3 ± 0.8), σw is the standard deviation of w, and cup and cdn are the average concentrations (over 30 min) of the trace gas in the updraft and downdraft reservoirs, respectively. The gas samples of N2O can then be analyzed by GC, or IR.
2.2.2.3. Aerodynamic gradient method (AGM)
This technique has been widely applied on a large variety of gases in the past fifty years, as it can rely on slower sensors than EC. Vertical profiles of temperature, wind speed and trace gas concentration (C) are used to calculate the flux (FC), according to:
where z is the height above the ground; the term KC represents the eddy exchange coefficient, and it is derived by similarity from the vertical profiles of wind and temperature (see e.g. Fowler and Duyzer 1989, Dyer and Hicks 1970, Wagner-Riddle et al 1996). In a very turbulent atmosphere (e.g. strong winds or convection during sunny days) the surface layer is well mixed and the gradients will be small. In a very stable atmosphere with hardly any turbulence (e.g. during nights with low wind speed) the differences between the measurement heights will be much larger.
2.2.2.4. Mass balance and plume methods
For source areas that have a clear border (a manure heap, a housing system, a test field), spatial integrating measurements are possible with either mass balance or plume measurement techniques.
Similar to the gradient technique, the mass balance technique has been used widely in the past few decades also for N2O (e.g. Denmead 2000): it uses concentration (C) measurements versus height, in combination with the vertical gradient of wind (U). The flux is calculated as U⋅C for all heights and integrated horizontally (e.g. Fowler and Duyzer 1987, Denmead et al 1998). This method is applied for finite sources that only stretch out about 4–5 times the height of the measurement tower in the upwind direction, and it is the bridge between micrometeorological techniques and plume measurements that rely on advection as well.
The plume method evaluates the concentration plume that originates at the source and is transported by the wind (Czepiel et al 1996, 2003, Trégourès et al 1999, Hensen and Scharff 2001). Typical distances between source and measurement transects are 50 m−2 km (figure 9). Wind speed, wind direction, turbulent parameters are needed to calculate the emission flux using a transport model. The plume technique can either use a mobile measurement system or an array of stationary samplers. Examples of a mobile system are shown in figures 9 and 10.
Figure 9. Mobile plume measurements: the van houses a fast N2O sensor and sonic anemometer and travels at a constant speed downwind of N2O plumes originating from various farm source.
Download figure:
Standard image High-resolution imageFigure 10. Typical trace of mobile plume measurements in an agricultural area using a TDL to detect N2O fluxes at 10 Hz recorded in spring on a 33 ha flat region in UK, containing crops, a small poultry farm, dung heaps and an organic farm. The average concentration increase was 2.6 ± 2.7 ppb (1 ppb = 1 ng l−1). Results suggest that for this period the N2O emission rate was 290 ng N2O m−2 s−1.
Download figure:
Standard image High-resolution imageBoth the plume and mass balance method can use relatively low cost sampling systems. With a focus on relevant sources (high emitters) relatively slow opto-acoustical sensors or GC analyses can provide the concentration data. In addition these methods require meteorological instrumentation. A dispersion model is needed to translate the measured concentration levels into emission levels. This can be a Gaussian model, but new backward Lagrangian models are now available that bring improvement on the modeling part of this type of emission evaluations (Loubet et al 2010).
No model is required when a tracer release is used (Scharff and Hensen 2009), provided that the tracer source distribution can sufficiently mimic the actual N2O source distribution.
2.2.2.5. Tall tower measurements
High resolution measurements at a single site with elevation over 100 m on a tower, a high building or a mountain can also be used to evaluate emissions. The measurements detect N2O passing the tower. Concentration peaks above background contain information on the sources upwind. With inverse modeling techniques, wind fields can be used to calculate the backward trajectories of air parcels that show where the air mass originated. On the European scale this technique is already used to evaluate country scale emissions these are then compared to the standard 'bottom up' method of emission inventories. Bergamaschi et al (2010) discuss how already national emission data has been updated based on this kind of analyses.
2.2.2.6. Boundary layer budget approach
Polson et al (2011) showed how the N2O budget for the UK can be evaluated using measurements from an airplane that flew around the UK, sampled airflow entering and exiting the country. During the flight air samples were collected into Tedlar® gas sample bags, to be analyzed when back on the ground for a range of gases, including N2O by TDL. Fluxes were calculated using inverse modeling techniques and compared to the UK national emission inventory. Smaller fluxes were reported by the bottom up inventory compared to the aircraft measurements for N2O, implying that the UK's annual N2O emission inventory using the International Panel on Climate Change methodology (IPCC 2006) may underestimate emissions.
3. Different research questions require different methods and sensors
The data produced by the observational systems described above are required to determine (i) which sources are important; (ii) the relative contribution of these sources; (iii) where and how to mitigate; (iv) how to extrapolate to the regional, national scale. It is important that appropriate tools are selected to address the above knowledge items.
Ecosystem or km2 scale budget studies aim to show what the large sources are and what their relative importance is (addressing (i) and (ii)). Comparison of source systems is based on annual area integrated data. The spatial integrating methods can provide this information.
In order to mitigate N2O emissions however insight is required into the processes that lead to or affect N2O exchange patterns (iii).
In order to understand the drivers of emission for example chambers are well suited. They can be used to evaluate different treatments in the field (e.g. Jones et al 2007) and in controlled environment laboratory studies (e.g. Sánchez-Martín et al 2008). Key is that all possible variables likely to influence the N2O flux are measured at the same time. Mechanistic models are data hungry and it is advisable to discuss data requirements with the modeler at the experimental design stage. High quality comprehensive data sets are the panacea for sensible model outputs. Widely used mechanistic models for N2O are DNDC (Li et al 1992) and DAYCENT (Parton et al 2001).
Upscaling to the national scale can be done in several ways. The most basic method to calculate annual N2O emissions from agricultural soils, the Tier 1, IPCC emission factor approach, is universally applicable, but with an uncertainty of >400% (IPCC 2006). The tall tower measurements linked with inverse modeling can provide a means for emission verification in an independent way. Across Europe a network of tall towers for GHG flux measurements is currently emerging in order to provide long-term observations of GHG and monitor change (Integrated Carbon Observation Systems (ICOS) www.icos-infrastructure.eu/).
Figure 11 shows how different measurement methods provide data on different spatial and temporal scales. The available measurement methods provide the knowledge at these different scales in time and space.
Figure 11. The role of different methods used in view of the spatial and temporal variability.
Download figure:
Standard image High-resolution image4. Final remarks and recommendations
In the scientific arena, in Europe, North America or new Zealand/Australia there is a clear trend towards the use of space integrating EC technique. This technique however need not be the first option for countries starting a programme of N2O research, or may not even be appropriate, as large uniform fields may not be characteristic of the agricultural landscape. When the starting point is a minimum in available research infrastructure, low cost techniques are certainly the first option.
Chamber, plume and gradient methods can be done low cost and can provide emission data on different source systems. Chambers are the most obvious choice to start with, they provide valuable information on N2O fluxes when used properly. It is important to follow guidelines on chamber design, sampling, flux calculation, replication in space and time etc.
Chamber and mass balance technique seem so simple that there is a danger to miss some of the warning signs that can be found in literature. Taking the latest recommendations for these methods into account however, might rapidly increase the price of the low cost setup and available budget might impose a limit on what is possible.
Chambers allow for off line sample analyses by GC, which can even be done abroad when conditions would not allow having a N2O monitor on the site or within the country. However, having an analyzer close to the measurement site would provide much faster information of temporal changes in flux rates and allows optimization of the measurement strategy (e.g. length of chamber closure, frequency of chamber measurements) in response to changed environmental or management conditions.
The most recent laser systems, top end of the market, are definitely the best option in terms of performance: stability, precision, accuracy. They will allow both for chamber and the much more demanding eddy covariance or tall tower observations to be carried out. Even though the initial price of latest generation IR systems (circa 100 kEuro) is about a factor of two above a complete GC system. However, they are becoming more competitive as the lower operational costs may pay back in the long run. With the laser spectrometers there is no need for carrier gases and only limited need for calibration gases. The latest laser spectrometers no longer need liquid nitrogen, and do not have the safety limitations of the radioactive GC–ECD sensors. Important is the need to use inlet filters since optical systems and especially the highly reflective mirrors in the measurement cell can get damaged by particles.
A limitation for field deployment of IR laser spectrometers will be power requirements. Small air samples (∼1 l) can be collected manually into gas tight bags or vials using a large syringe or small vacuum pump from chambers, mass balance, gradient or stationary plume measurements. For eddy covariance however, the measurement cell has to be flushed at a high flow rate whilst maintaining low pressure in the cell. In practice that requires 1–4 kW pumps to be used. Running these systems on, for example, solar or wind power can be a problem. It is to be expected that in the coming years low power open path systems will be developed that can circumvent these limitation.
In fact the choice what to do right now is not so much what instrument to use but what method to use. That mainly depends on the questions asked. For a regional emission validation, use the tall tower (for example a telecommunication tower) with inverse modeling technique. For process understanding small chambers and for field scale evaluation or multiple plot emission surveys the mass balance, gradient, plume or eddy covariance methods are suitable approaches.
With the current speed of developments in optical measurement techniques low cost versions of the laser systems will become available that might cost 5–20 kEuro but have a 10 or 100 fold reduction of the resolution in concentration. These systems would be able to do chamber measurements or mass balance/plume measurements close to high emitters, but not allow for the micromet or tall tower techniques.
Acknowledgments
We wish to thank our national funding authorities (Natural Environment Research Council (NERC) and—Department for Environment, Food and Rural Affairs (DEFRA) in the UK and government funding in the NL) for support through several contracts over the years; and the EU for financing collaborative research through the FP7 integrated projects CarboEurope IP, Nitroeurope IP and before that GreenGrass and Midair.