ABSTRACT
Crystalline silica (SiO2) is recurrently identified at the percent level in the infrared spectra of protoplanetary disks. By contrast, reports of crystalline silica in primitive meteorites are very unusual. This dichotomy illustrates the typical gap existing between astrophysical observations and meteoritical records of the first solids formed around young stars. The cometary samples returned by the Stardust mission in 2006 offer an opportunity to have a closer look at a silicate dust that experienced a very limited reprocessing since the accretion of the dust. Here, we provide the first extended study of silica materials in a large range of Stardust samples. We show that cristobalite is the dominant form. It was detected in 5 out of 25 samples. Crystalline silica is thus a common minor phase in Stardust samples. Furthermore, olivine is generally associated with this cristobalite, which put constraints on possible formation mechanisms. A low-temperature subsolidus solid–solid transformation of an amorphous precursor is most likely. This crystallization route favors the formation of olivine (at the expense of pyroxenes), and crystalline silica is the natural byproduct of this transformation. Conversely, direct condensation and partial melting are not expected to produce the observed mineral assemblages. Silica is preserved in cometary materials because they were less affected by thermal and aqueous alterations than their chondritic counterparts. The common occurrence of crystalline silica therefore makes the cometary material an important bridge between the IR-based mineralogy of distant protoplanetary disks and the mineralogy of the early solar system.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
Silicates are the main building blocks of Earth-like planets. During the last two decades, considerable advances were accomplished to characterize silicates in disks around forming stars. From the Infrared Space Observatoryʼs crystalline revolution (Jäger et al. 1998) to the results of the Spitzer Space Telescope, the diversity of silicates detected around AGB, Herbig Ae/Be, and T Tauri stars continuously increased. Nonetheless, despite these advances, it is still challenging to bridge the gap between astrophysical observations and the mineralogy of the most primitive objects of the solar system, nowadays collected in the vicinity of the Earth. A good example of this gap is the detection of crystalline and amorphous silica around T Tauri stars (e.g., Bouwman et al. 2001; Honda et al. 2003; van Boekel et al. 2005; Lisse et al. 2009; Sargent et al. 2009; Juhàsz et al. 2010). While the total amount of silica is estimated to be 2%–10% (in cool components) in these infrared spectra, crystalline silica is a very unusual mineral in primitive objects studied in the laboratory.
In this context, the first sample return of cometary materials, the Stardust mission, raised considerable expectations from astrophysicists, mineralogists, and cosmochemists. Considered as a reservoir of pristine material accreted from an extended area of the protoplanetary disk, comets could help to reconcile astrophysical and laboratory observations. The Stardust mission captured and returned cometary grains from the coma of comet 81 P/Wild 2 (Brownlee et al. 2006). The Wild 2 particles contain a high diversity of crystalline refractory material such as fragments of chondrules and calcium–aluminum-rich inclusions (CAIs), bearing the signature of high-temperature processes (e.g., Zolensky et al. 2006; Leroux et al. 2008a; Nakamura et al. 2008; Simon et al. 2008; Chi et al. 2009; Joswiak et al. 2009, 2012). They also contain a fine-grained and less refractory material also deeply thermally modified during the hypervelocity impact at 6.1 km s−1 into the aerogel collector medium (e.g., Leroux et al. 2008b; Roskosz et al. 2008; Leroux et al. 2009).
The crystallinity of the Stardust samples is estimated to be at least 50% (Ogliore et al. 2009; Wesphal et al., 2009; Stodolna et al. 2012). Crystalline material includes a wide diversity of minerals formed under different conditions, suggesting a wide range of processes leading to the formation of crystalline silicates (Zolensky et al. 2008a). The mineralogy is dominated by olivine and pyroxene, but other minerals have been identified. In particular, crystalline silica was occasionally reported. Tridymite was found in one sample (Zolensky et al. 2006; Matrajt et al. 2008; Joswiak et al. 2012). Cristobalite was found in tracks 80 and 41 (Joswiak et al. 2012; Stodolna et al. 2012 respectively). However, no clear characterization or interpretation was proposed. Here, we provide the first extended study of silica materials in a large range of Stardust samples. We show that the mineralogy of this cometary material is more compatible with astrophysical observations than the mineralogy of chondritic materials.
2. SAMPLES AND METHODS
The Stardust samples were allocated by the Johnson Space Center, Houston, Texas, USA (Zolensky et al. 2008b). For transmission electron microscopy (TEM) studies, the samples need to be electron transparent (typically a thickness of 100 nm or less is required). Samples were prepared by ultramicrotomy after particle extraction from dissected aerogel and embedding into low-viscosity epoxy (Zolensky et al. 2008b) or by using the acrylic embedding method (Matrajt & Brownlee 2006). Samples were then studied by TEM at the electron microscopy center of the University of Lille, France. Conventional bright and dark field TEM was used for direct imaging. Structural data were obtained by electron diffraction (ED) and chemical data by microanalysis using energy dispersive X-ray microanalysis (see Leroux et al. 2008b for more details about the analytical procedure).
The composition of pyroxenes and olivines is reported as the molar fraction of the different endmembers of these two solid solutions. Olivines have two endmembers: forsterite (Fo–Mg2SiO4) and fayalite (Fa–Fe2SiO4). For instance, an olivine containing 80 mol.% of forsterite and 20 mol.% of fayalite will be labeled Fo80. Similarly, the pyroxenes analyzed here have three endmembers: enstatite (En–Mg2SiO6), wollastonite (Wo–Ca2SiO6), and ferrosilite (Fs–Fe2SiO6). A pyroxene containing 50 mol.% of enstatite, 40 mol.% of wollastonite and 10 mol.% of ferrosilite will be labeled En50Wo40Fs10.
3. RESULTS
Out of the 25 Stardust samples studied, cristobalite was found in 5 samples, namely, C2044,2,41,3,6; C2009,20,77,2,36; C2009,20,77,2,39; C2009,20,77,5,18; and C2009,20,77,4,1. Interestingly, these five samples exhibit some regularities but also a significant variability in terms of mineralogy that we present in the following sections.
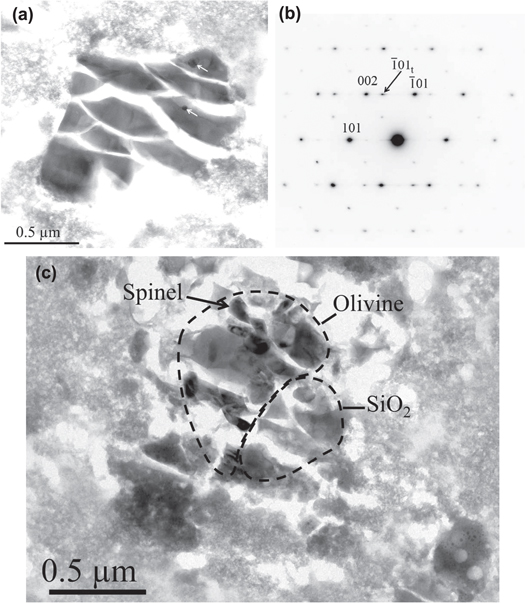
Sample C2009,20,77,5,18 contains a number of cometary fragments distributed throughout the compressed aerogel. This dispersion is due to the fragmentation of the incoming particle during the impact. Most of the fragments are sub-micron in size and consist of strongly thermally modified material for which the bulk composition is close to the solar value for major elements (Fe, Mg, S, and excluding Si because of the aerogel contamination). The classical interpretation of such material is that they were flash-melted and mixed with molten aerogel during the collection and subsequently quenched as a glass. The extensive impregnation of the cometary material by aerogel prevents any identification of small (typically <200 nm) indigenous grains of SiO2 if initially present. However, in addition to this glassy material, sample C2009,20,77,5,18 also contains crystalline fragments. Three equilibrated polycrystalline assemblages were found, dominated by olivine (Fo93, Fo87, and Fo61–65, respectively) and containing minor Ca–pyroxene and Fe–Cr–Al-spinel. Several isolated single crystal fragments were also identified: pyroxene (En52Wo38Fs10, 1 grain), forsterite (1 grain), enstatite (2 grains), anorthite (CaAl2Si2O8, 1 grain), and crystalline silica (2 grains). One silica grain, a single crystal of 1.5 μm in size, was well separated from the other fragments (Figure 1(a)). The grain contains two small inclusions of iron sulfide for which the ED pattern is compatible with pyrrhotite. ED (Figure 1(b)) indicates that the mineral is the metastable low-temperature tetragonal α form of cristobalite, which in turn is a high-temperature silica polymorph. The grain is twinned on the plane (101). Typically, such crystal defects are due to the transition from the high-temperature β form to the low-temperature α form that occurs at 230 °C. Contrasting with this large and isolated grain, the second silica grain is in close association with a polycrystalline olivine (Fo61–65) and a small Fe–Cr–Al-spinel grain (Figure 1(c)). The grain size is about 500 nm in diameter, while the polycrystalline olivine in contact is about 1.5 μm in size. Here, again, the ED patterns indicate that the mineral is cristobalite and no twinning was observed.
Figure 1. Sample C2009,20,77,5,18. (a) Single crystal of cristobalite in the compressed aerogel, bright field TEM image. The white arrows indicate the presence of small iron sulfur inclusions. The shard-like aspect is due to ultramicrotomy sectioning (brittle behavior of the cristobalite grain). (b) Corresponding electron diffraction pattern, indexed as cristobalite. The pattern contains additional spots (one is arrowed and indexed as "t") related to the twinning on the (101) plane. (c) Bright field TEM image of an aggregate of cristobalite and olivine (Fo61–65). Dashed lines mark the grain boundaries as guide for the eyes.
Download figure:
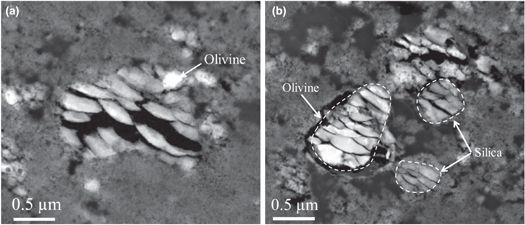
Standard image High-resolution imageThe two Stardust samples C2009,20,77,2,36 and C2009,20,77,2,39 are two ultramicrotomy slices from the same particle. However, they are not adjacent slices. As in the previous sample, a large number of cometary fragments were found. Many of them were strongly damaged during the impact. However, several crystalline phases survived. Several olivine grains (Fo72, Fo83, Fo100), one pyroxene grain (En70Wo09Fs21), and three crystalline SiO2 grains were identified. The largest silica particle (1.2 μm in size) is in contact with a small olivine grain (200 nm in diameter, Fo84; Figure 2(a)). Another small fragment (∼200 nm) is associated with an olivine grain (Fo88) and with an impact melt pocket. The third crystalline SiO2 grain is isolated into the compressed aerogel. It has an elongated shape (500 × 300 nm) and was identified as cristobalite by ED. It contains numerous twins along the plane (101). In addition to these three cristobalite fragments, three other amorphous SiO2 grains were unambiguously identified. Several features make them fundamentally different from compressed or molten aerogel. The compositions of the grains are almost pure SiO2, with minor Fe, Al, P, and K (≈0.7, 0.5, 0.4, and 0.1 at.%, respectively), suggesting that the amorphous silica is a flash-melted cristobalite grain during the impact. Indeed, the presence of a small amount of cation substitution is frequent in natural cristobalite (Smith & Steele 1984), while the flight aerogel contain impurities at the ppm level (Lanzirotti et al. 2008; Stephan et al. 2008). Furthermore, no vesicles were observed, whereas molten aerogel always exhibits large vesicles (see Leroux et al. 2008b for details). Finally, the interface with the surrounding compressed aerogel is relatively sharp. Turning to the mineralogical associations, two of them are closely associated with a large single crystal olivine (1 μm, Fo54; Figure 2(b)) and small melted patches resulting from the melting and the mixing of a fine-grained material with aerogel. The last grain was isolated in the compressed aerogel (1 μm in size). Finally, we note that it is not possible to fully establish whether these grains were initially amorphous or were flash-melted during the collect or amorphized under the electron beam during TEM work (SiO2 polymorphs are highly sensitive to irradiation).
Figure 2. Dark field scanning TEM images from C2009,20,77,2,36 and C2009,20,77,2,39. (a) Relatively large crystalline SiO2 grain in association with olivine (Fo84, arrowed) within the compressed aerogel. (b) Two amorphous SiO2 grains, in close association with olivine (Fo54) and a patch of impact melt.
Download figure:
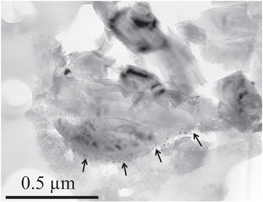
Standard image High-resolution imageSample C2044,2,41,3,6 is essentially a large impact melt (several μm in diameter). A cristobalite grain (1 μm in diameter) was found at one edge of this impact melt (Figure 3). One side of the grain displays clear evidence of interaction with melted aerogel mixed together with a small amount of cometary material. This rim likely corresponds to the hemispherical caps described around particles moving into the aerogel at high speed (Barrett et al. 1992).
Figure 3. Bright field TEM image of a cristobalite grain surrounded by a dense silica-rich layer of melted aerogel (arrows) in sample C2044,2,41,3,6. The dense silica-rich cap also contains a few tiny Fe–sulfide inclusions resulting from the interaction of the Wild 2 particle with aerogel.
Download figure:
Standard image High-resolution imageFinally, sample C2009,20,77,4,1 contains only four crystallite fragments: one olivine (Fo85), one low-Ca pyroxene (En84), and two SiO2crystalline grains (sizes 300 and 400 nm). The cristobalite structure was confirmed by the ED pattern on one of these two grains.
4. DISCUSSION
Wild 2 particles contain a significant abundance of silica grains. An order of magnitude of this abundance is difficult to establish. Indeed, studies by TEM are not well adapted to derive modal abundances. Relatively poor statistics result from the small amount of matter accessible by this investigation technique. Thermal effects associated with the high-speed capture of the Wild 2 grains in the aerogel also blurred the bulk mineralogy of the pristine cometary materials. As a consequence, the mineralogical signature and abundances are likely biased (see Leroux 2012 for a detailed discussion on this topic). In particular, small silica particles (>200 nm) are undetectable after their interaction with silica aerogel. With these cautions in mind, when present in the Stardust samples, silica phases were detected in one-fifth of the studied samples and represent about 10% of the comet material present in these samples. Therefore, about 2% of crystalline silica is probably present in the returned cometary samples. This estimate is a lower limit but is already higher than in most extraterrestrial material studied so far.
Silica phases are quite unusual in extraterrestrial material. Silica polymorphs (mainly cristobalite and tridymite) are found as minor minerals in differentiated igneous material such as lunar samples (e.g., Mason 1972), Martian meteorites (e.g., Leroux & Cordier 2006), and achondrites (e.g., Benzerara et al. 2002). They typically form during the late stage of the crystallization sequence from a highly differentiated melt. In chondrites, silica polymorphs (quartz, cristobalite, tridymite, and amorphous) are reported in enstatite chondrites (e.g., Kimura et al. 2005). In ordinary and carbonaceous chondrites, silica polymorphs have only been observed in unusual silica-rich chondrules or as magmatic inclusions (e.g., Brigham et al. 1986; Hezel et al. 2006). To our knowledge, silica grains have not been identified in the fine-grained matrix that cements chondrules. In this context, the relatively frequent occurrence of silica grains in Stardust cometary samples is an important observation that may suggest some mineralogical differences between cometary and meteoritic materials, the bulk composition of these materials being solar in both cases.
The presence of a significant amount of silica in the Stardust samples may result from three different pathways: (1) equilibrium or disequilibrium condensation from a gas, (2) crystallization from a melt, and (3) solid–solid reactions at even lower temperatures.
1. Thermodynamic models suggest that silica does not condense at equilibrium in stellar environments from a solar gas composition (e.g., Ebel & Grossman 2000). However, when the Mg/Si ratio is less than 1, both equilibrium and non-equilibrium condensation might lead to the formation of the assemblage enstatite + silica (e.g., Petaev & Wood 1998; Ebel & Grossman 2000; Ferrarotti & Gail 2001). Such direct condensation is likely at the origin of presolar silica grains (Nguyen et al. 2010; Floss & Stadermann 2012; Haenecour et al. 2013). In the studied Stardust samples, silica is systematically associated with Fe-rich olivines rather than with Fe-free pyroxene (enstatite). Such an association is not predicted from available condensation models, even in the O/Si zone of massive stars (Ebel & Grossman 2000). For this reason, the silica grains observed in Stardust samples are unlikely formed by direct condensation. This does not imply, however, that presolar silica grains cannot be found in the Stardust collection.
2. A significant fraction of Stardust grains have an igneous origin (e.g., Leroux et al. 2008a; Nakamura et al. 2008; Zolensky et al. 2008a). As pointed out by Hezel et al. (2006), there are different ways to produce silica from a highly differentiated liquid during crystallization either in closed systems or during interactions with a SiO-rich gas. However, the occurrence of silica in extraterrestrial objects is unusual and restricted to exotic chondrules and differentiated bodies (Mars, Moon...). It is not clear whether the Stardustʼs silica grains can result from the crystallization of a melt. Based on mass-balance considerations, it is unlikely that stardust samples could contain such a large amount of igneous silica, whereas chondrites, which do have an igneous component, rarely host silica. Nonetheless, the association of silica with anorthite in one sample (C2009,20,77,5,18) may reflect an igneous origin for a fraction of the silica grains found in Stardust samples. Other mechanisms discussed here would hardly produce such a silica- and calcium-rich assemblage from a typical protoplanetary dust (with a ratio of Si/Mg close to unity). This could also be consistent with a crustal origin (after the disruption of a highly differentiated body) as recently suggested from Spitzerʼs observation (Lisse et al. 2009).
The detection of silica in protoplanetary disks is often interpreted as the result of the partial melting of pyroxenes (e.g., Sargent et al. 2009). Partial melting of pure enstatite could indeed lead to the formation of the assemblage silica + forsterite. However, the situation is dramatically different in most of natural environments because pyroxenes generally contain a significant amount of calcium and aluminum (at least at the wt.% level or even more). These two elements cannot be accommodated at a significant level in the crystal structure of olivine (typically less than 0.2 wt.% for Ca). The stardust pyroxenes and olivines are not an exception to these rules. In this context, the actual phase diagram for natural pyroxenes shows an extremely limited compositional area where partial melting of pyroxene could lead to the formation of both olivine and silica (Huebner & Turnock 1980). In the Fe-rich region (Fe/Fe + Mg >60%), silica can form from partial melting, but the residual melt does not crystallize as pure olivine. Furthermore, olivines and pyroxenes detected in our samples have much less iron than what would be required to observe such a transformation. Conversely, the partial melting of the Mg-rich region of the phase diagram never produces pure crystalline silica (Huebner & Turnock 1980). As a conclusion, the composition of olivines and pyroxenes in the stardust samples does not support the formation of cristobalite as a result of the partial melting of preexisting pyroxenes.
3. The processes involving gases and melts hardly produce significant amount of silica. They also generally lead to the formation of coarse-grained materials. Instead, silica could result from the subsolidus crystallization of an amorphous precursor by solid–solid reactions. Our recent experimental studies show that an abrupt change of mineralogy occurs when amorphous silicate dust is annealed below and above its glass transition temperature (Roskosz et al. 2009, 2011). Below this temperature, olivine is the dominant crystalline phase (Fabian et al. 2000; Thomson & Tang 2001). This also holds true when the starting amorphous material is markedly enriched in silica compared to olivine and should, under equilibrium conditions, form pyroxenes. In this situation, the residual material readily crystallize as cristobalite at anomalously low temperatures if annealing duration is long enough (Roskosz et al. 2009; Matsuno et al. 2012; Day et al. 2013). Conversely, above the glass transition temperature, where the amorphous material shares the dynamics of highly viscous melts, phases predicted by equilibrium phase diagrams become dominant (typically pyroxenes are formed). These results demonstrate that a low temperature, subsolidus crystallization of amorphous silicates always leads to the formation of olivine and that silica is a natural byproduct of this metastable crystallization. This crystallization is the consequence of the high mobility of divalent cations in amorphous silicates below the glass transition, where the mobility of silicon is vanishingly small (Roskosz et al. 2006). In protoplanetary disks, the phase transformation seen by most of the silicate dust (beside the dust present in the inner zone where vaporization occurs) occurs at around 1000 K. At this temperature, no melt is produced, but the assemblage olivine + silica is obtained along such a subsolidus route. With this assemblage being metastable, it should be destroyed by any high-temperature processes at work in the inner solar system and by aqueous alteration in chondritic parent bodies. This is certainly why they are rarely observed in meteorites but rather are observed in the returned comet samples.
5. CONCLUSION
The solid-state route both accounts for the recently established presence of olivine and silica grains in disks and in the fine-grained material found in Stardust samples. One of the main conclusions of the stardust mission is that the mineralogy of Stardust samples does not drastically differ from the meteoritical record (see Brownlee 2014 for a recent review). The frequent occurrence of silica grains contradicts this assumption. It shows that the early stage of dust crystallization, documented by astrophysical observation and lost in chondrites, was preserved in cometary materials. Finally, our study shows that more systematic and statistical determinations of mineral compositions and assemblages could unravel other subtle differences between cometary and meteoritical materials. It would help to merge astrophysical and mineralogical descriptions of the protosolar silicate dust in a more consistent way than what is presently achievable from chondritic materials.
The authors thank K. Nakamura-Messenger, G. Matrajt, and D. Brownlee for the preparation of high-quality ultramicrotomed TEM Stardust samples. They are grateful for support by Centre National d'Etudes Spatiales (CNES) during this investigation period of the Stardust samples. The TEM national facility in Lille (France) is supported by the Conseil Regional du Nord-Pas de Calais, the European Regional Development Fund (ERDF), and the Institut National des Sciences de l'Univers (INSU, CNRS).