Abstract
Fe-doped TiO2 catalyst was prepared by the hydrothermal method. The resulting nanopowders were characterized by x-ray diffraction, transmission electron microscopy and Raman and UV-visible spectroscopies. The photocatalytic activity of the Fe-doped TiO2 was tested by decomposition of methylene orange with a concentration of 10 mg l−1 in aqueous solution. The obtained results showed that methylene orange was significantly degraded after irradiation for 90 min under a halogen lamp and sunlight. The doping effect on the photocatalytic activity of the iron-doped catalyst samples are discussed.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
One of the most intriguing application areas of titanium dioxide is photocatalytic activity for environmental protection, since it can decompose a large number of organic and inorganic pollutants [1, 2]. However, one disadvantage of TiO 2 is that the energy band gap is approximately 3.2 eV, therefore UV illumination is necessary for photoactivating this semiconductor. Another disadvantage of TiO 2 is that charge carrier recombination occurs within nanoseconds and the photocatalytic activity is low.
Attempts to improve the performance of TiO 2 as a photocatalyst and to extend its light absorption range have primarily been concerned with the effect of dopants [3, 4]. A wide range of metal ions, in particular transition metal ions, have been used as dopants for TiO 2, and their effects on the properties of the doped samples have been reported [5–10]. It is not easy to compare the results reported for doped samples due to a variety of experimental conditions and preparation methods. The photocatalytic activity of the doped TiO 2 photocatalyst depends on the nature and concentration of the dopant ion.
The hydrothermal method has already been applied to synthesize nanosized materials, since products prepared by this method have a good crystalline phase, which benefits the thermal stability of the nanosized materials. Kasuga et al [11] treated TiO 2 in a 10 M NaOH aqueous solution for 20 h at 150 °C without the need for a template, and nanotubes with 8 nm diameter and 100 nm length were obtained. Grimes and co-workers [12] obtained TiO 2 nanotubes by using the same hydrothermal process for 30 h at 180 °C. To the best of our knowledge, TiO 2 doped with metal via such a method has not been reported.
In this paper, Fe-doped TiO 2 catalysts were prepared by the hydrothermal method. The resulting nanopowders were characterized by x-ray diffraction (XRD), transmission electron microscopy (TEM) and Raman and UV-visible spectroscopies. The photocatalytic activity of the Fe-doped TiO 2 was tested by decomposition of methylene orange with a concentration of 10 mg l −1 in aqueous solution. This method is quite simple and can be used to prepare a large number of products.
2. Experimental
2.1. Synthesis of iron-doped TiO2
2 g of commercial TiO 2 powders (Merck, Germany) and 3 mg of Fe 2 O 3 (Merck, Germany) (weight ratio of Fe to Ti was 0.1%) were dispersed in 50 ml of a 10 M NaOH solution in a glass cup by ultrasonic wave (35 kHz, 100 W) for 30 min. The sonicated solution was then moved to a teflon vessel and put in a stainless steel autoclave to carry out hydrothermal treatment at 140 °C for 14 h. The stainless steel was then opened at room temperature and the precipitates were separated and washed repeatedly with 0.1 M HCl aqueous solution and deionized water until the pH of the washing solution dropped lower than 7. The final as-prepared pale violet product was dried under vacuum at 80 °C for 12 h. Then the product, 0.1% Fe:TiO 2, was calcined at 600 °C for 1 h in air.
Using the same hydrothermal process, we prepared 0.2% Fe:TiO 2 and 0.3% Fe:TiO 2. The more iron ion that was doped, the darker the colour of the product.
2.2. Characterization and measurement
The morphology, dimensions and microstructure measurements of the samples were performed using a JEOL-2010F high resolution transmission electron microscope (HR-TEM). The crystalline phases of the obtained samples were characterized by using a Siemens D-5005 x-ray powder diffractometer (XRD) with monochromatized Cu-Kα irradiation (λ=1.54056 Å). The Raman spectra were recorded on a Nicolet spectrometer equipped with an optical microscope at room temperature. For excitation, the 514.5 nm line from an Ar + ion laser (Spectra Physics) was focused, with an analysing spot of about 1 μm, on the sample under the microscope. UV-Vis spectra were measured by a Scan UV-Vis spectrophotometer (Varian, Cary 500). The specific surface area of the samples was determined by nitrogen adsorption at 77 K (Quantachrome NOVA 1000-TS, USA).
The photocatalytic activity was evaluated by measuring the decomposition of the aqueous solution of methylene orange (with a concentration of 10 mg l −1) under visible light and sunlight irradiation. Visible light irradiation was carried out using a 300 W halogen lamp. The reactor was equipped with water circulation in the outer jacket in order to maintain a constant temperature. Prior to irradiation, the suspensions were magnetically stirred in the dark for 30 min to ensure the establishment of an adsorption/desorption equilibrium. Then the solution was filtered to remove any particles. The resulting solution was analysed by recording variations in the absorption in the UV-visible spectra of methylene orange using a Shimadzu 1601-PC UV-Vis spectrometer.
3. Results and discussion
3.1. Microstructure of materials
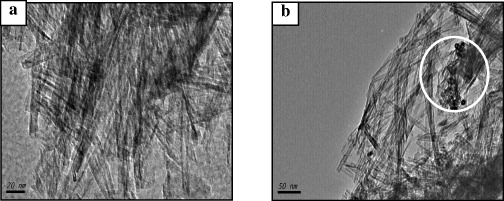
Figure 1 shows HR-TEM images of the samples (TiO 2 prepared by the hydrothermal method (a) and 0.3% Fe:TiO 2 (b)) calcined at 600 °C. Figure 1(a) shows the morphology of hollow tubular structures with outer diameters of 10 nm and lengths of 500 nm. Figure 1(b) indicates that the morphology of Fe-doped TiO 2 is similar to the TiO 2 sample without doping. We also observed a few particles Fe 2 O 3 sticking to the outside of the TiO 2 nanotube and the particle size is about 15 nm. This result demonstrated that the reaction happened when TiO 2 had been doped by Fe 2 O 3.
Figure 1 HR-TEM images of the samples: (a) TiO 2 prepared by the hydrothermal method and (b) 0.3% Fe:TiO 2.
3.2. Properties and structures of photocatalysts
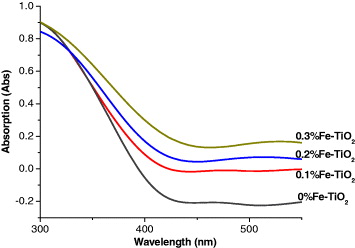
UV-Vis spectra of Fe-doped TiO 2 with different Fe 3+ doping concentrations are presented in figure 2. It is apparent that the UV-Vis spectra of all the Fe-doped samples have extended a red shift and increased absorbance in the visible range. With increasing Fe 3+ doping concentration, the absorbance increases. The red shift of the absorption edge in Fe-doped titania has been attributed to the charge-transfer transition between the iron ion d-electrons and the TiO 2 conduction or valence band [13]. It has been reported that metal doping could form a dopant energy level within the band gap of TiO 2. The electronic transitions from the valence band to the dopant level or from the dopant level to the conduction band can effectively red shift the band edge adsorption threshold.
Figure 2 The UV-Vis spectra of Fe-TiO 2 with different Fe 3+ doping concentrations.
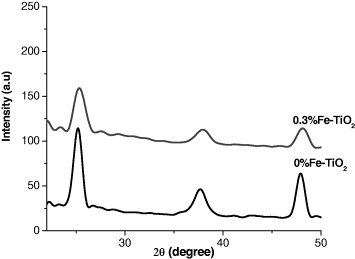
Figure 3 represents x-ray diffraction patterns for pure TiO 2 and TiO 2 doped with 0.3% iron ion. These samples exhibit only patterns assigned to the well crystalline anatase phase. Due to very low Fe content in the Fe-doped TiO 2, any crystalline phase containing Fe could not be observed by XRD. It is noteworthy that no iron oxide or Fe x TiO y peak could be observed in the XRD spectra. We think all the iron ions were incorporated into the structures of titania and replaced titanium ion or located at interstitial site.
Figure 3 X-ray diffraction patterns for pure TiO 2 and 0.3% Fe:TiO 2.
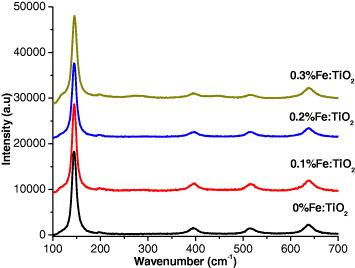
Figure 4 shows the Raman spectra of different samples. Raman peak at about 146 cm −1 is observed for all the samples, which is attributed to the main Eg anatase vibration mode. Moreover, vibration peaks at 199 cm −1 (Eg, weak), 399 cm −1 (B1 g), 516 cm −1 (A1 g) and 640 cm −1 (Eg) are presented in the spectra for all samples, which indicates that anatase TiO 2 crystalline are the predominant species. Furthermore, there is no peak attributed to the iron oxide observed, which is consistent with the results of XRD patterns.
Figure 4 Raman spectra of the Fe-doped TiO 2 samples.
3.3. Photocatalytic activities of photocatalysts
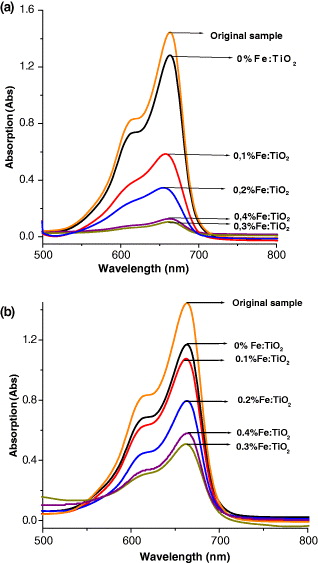
In the present study, the samples that were doped at various ratios of iron to titania were tested by the photo-oxidation of methylene orange (MO). Figure 5 shows the UV-visible absorption data on the gradual decrease in MO concentration under visible light (figure 5(a)) and sunlight (figure 5(b)) after an irradiation time of 90 min.
Figure 5 UV-Vis absorption spectra of MO on Fe-doped TiO 2 samples under sun light (a) and a halogen lamp (b).
It can be seen that the more iron ion that was doped, the greater the increase in the rate of degradation of MO. It is worth noting that the amount of dopant iron ion is very important to the level of photoactivity. However, the degradation rate of MO on sample 0.4% Fe:TiO 2 was slightly weaker than on the sample 0.3% Fe:TiO 2. It can be explained that the increase in dopant ion can increase the rate of the recombination of electron-hole pairs, because iron ions play the role of recombination centres. Under sunlight, the decomposition of MO is faster than that under a halogen lamp. Because the sunlight contains some proportion of UV-radiation (about 5%), these results indicated that Fe-doped TiO 2 has catalytic ability in the visible region.
3.4. Discussion of the physical mechanism
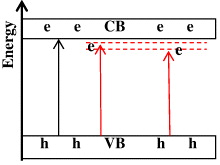
The effects of doping iron ions on the photocatalytic activity of titania can be explained as follows. On the one hand, the doping Fe 3+ induces the narrowing of the band gap of TiO 2. Cong et al [14] reported that in the metal ion-implanted TiO 2 the overlap of the Ti d-orbital of TiO 2 and the metal d-orbital of the implanted metal ions leads to the narrowing of the band gap of the material. Moreover, the dopant Fe 3+ ion induces the formation of new states close to the conduction band (figure 6). Therefore the doping by iron ions greatly improves the photocatalytic activity in the visible light region [15]. On the other hand, it inhibits the recombination of the photogenerated electron and hole. The Fe ions with a suitable concentration can trap the photogenerated electron, which enhances the utilization efficiency of the photogenerated electron and hole.
Figure 6 Schematic diagram of the mechanism of Fe 3+ doping on TiO 2.
4. Conclusion
In this study, photocatalysts TiO 2 doped with Fe 3+ are successfully prepared by the hydrothermal process. Fe 3+ can be incorporated into the lattice of titania. This is tested by XRD and Raman spectra. The UV-Vis spectra of the iron doped samples shift remarkably to the visible light region and the absorption is enhanced significantly. The photocatalytic activities of the samples are investigated under both sunlight and halogen lamp irradiation. The probable physical mechanism of the doping effect is proposed. It is presumed that the presence of Fe 3+ ion leads to the narrowing of the band gap and improves the photocatalytic activity in the visible light region.
Acknowledgment
This work is supported in part by the Grant-in-Aid for Scientific Research from the Ministry of Education and Training, Vietnam (Code B2010.28.29, 2010-2011).