Abstract
In recent years, the antimicrobial nanofinishing of biomedical textiles has become a very active, high-growth research field, assuming great importance among all available material surface modifications in the textile industry. This review offers the opportunity to update and critically discuss the latest advances and applications in this field. The survey suggests an emerging new paradigm in the production and distribution of nanoparticles for biomedical textile applications based on non-toxic renewable biopolymers such as chitosan, alginate and starch. Moreover, a relationship among metal and metal oxide nanoparticle (NP) size, its concentration on the fabric, and the antimicrobial activity exists, allowing the optimization of antimicrobial functionality.
Export citation and abstract BibTeX RIS
Introduction
In the last 20 years, pathogenic bacteria have developed resistance to almost all the commercially available antibiotics, and the number of new antibiotics expected to enter the market is limited [1]. Thus, searching for new antibacterial agents is a priority for pharmaceutical companies and researchers. Recently, novel antimicrobial agents have been developed using nanoscale materials. Compared to classic antibiotics, these materials have a lower propensity to induce high-level, single-step resistance mutation due to their multi-targeted mechanism of action, high surface area to volume ratio, and unique chemical and physical properties [2–4]. Numerous types of nanomaterials with antimicrobial properties such as copper [5, 6], zinc [7], titanium [8, 9], magnesium [10], gold [11], chitosan [12] and alginate [13] have been developed in recent years. However, among all, silver nanoparticles (AgNPs) have proved to be the most effective against bacteria, viruses and eukaryotic microorganisms and are being exploited in medicine for burn treatment, dental materials, metal coating, textile fabrics, water treatment and sunscreen lotions [1]. Moreover, silver has proved to have low toxicity to human cells, high thermal stability and low volatility [14]. It is known that the size, shape and crystalline structure of AgNPs affect their toxicological impact on microorganisms. However, the mechanism of bactericidal actions of AgNPs is still not fully elucidated, particularly because most of the available toxicity data are obtained in water or cell culture media, which do not reflect the complex environment inside living organisms [15]. Nowadays, the prevailing paradigm suggests a combination of various factors: (i) Nanoscaled direct interactions between NPs and cell membranes affect their permeability and are followed by a cascade of intracellular reactions, including DNA condensation; (ii) Silver ions reacting with thiol groups of cellular proteins interferes with the bacterial respiratory chain; (iii) Extracellular and intracellular generations of reactive oxygen species have resulted in membrane lipid and DNA damage [14]. Antimicrobial finishing of textiles for biomedical purposes has become an important area of research and one of the fastest growing sectors of the textile market. The global Antimicrobial Coatings Market's worth in 2012 is $1.5 billion and is estimated to reach $2.9 billion by 2018, growing at a compound annual growth rate of 11.8% from 2013 to 2018 under normal conditions [16, 17]. In general, the activity of antimicrobial finishes in textiles can be classified as biocidal or biostatic [18]. While the biocides include agents that kill microorganisms, the biostatics inhibit the microorganisms' growth. Antimicrobial textiles commonly use biocides, such as metal nanoparticles (or their salts), quaternary ammonium compounds, poly(hexamethylene biguanide), triclosan and chitosan, as active agents. These agents are either incorporated into the fibers during extrusion or attached to their fiber surface during finishing [19]. However, the definitions of 'bacteriostatic' and 'bactericidal' do not enclose two pure categories of antimicrobial agents that exclusively kill bacteria or that only inhibit growth. Within 18–24 h after the test, bactericidal agents usually fail to kill every organism, especially if the inoculum is large, and bacteriostatic agents kill some bacteria [20]. Moreover, the in vitro microbiological determination of an antibacterial agent in textiles is also influenced by growth conditions, bacterial density, test duration, extent of reduction in bacterial numbers, fabric shape, morphology and type of material. The most effective methods for testing the efficacy of fabrics that contain antimicrobials are the AATCC 100 and the AATCC 147 from the American Association of Textile Chemists and Colorists. The first test determines both the bacteriostatic activity as well as the bactericidal activity; the second test detects bacteriostatic activity of diffusible antimicrobial agents on treated textile materials by determining a zone of inhibition. However, during the preparation of the manuscript and the compilation of table 1, it became evident that there was a large variety of different tests that could be utilized to determine the fabrics' antimicrobial activity as well as a lack of information about the methodologies utilized to determine whether an antibacterial agent is bactericidal or bacteriostatic. Most antibacterial textiles are better described as potentially being both bactericidal and bacteriostatic, but in this review, they have been considered 'bactericidal' textiles only when able to kill more than 99.9% of the inoculum.
Table 1. Antimicrobial nanomaterials applied on textiles.
| Textile fabric and fiber [Nanomaterial] | NPs average size (nm) [Concentration] | Method | % Bacterial reduction [Strain] | % Bacterial reduction after washing [Strain] (Washing cycles) | Reference |
|---|---|---|---|---|---|
| Acrylic | |||||
| [Ag] | 1–7 | Photocured Carboxymethyl Starch NPs | 20* [S. aureus] 15* [E. coli] | 1* [S. aureus] (15) 0.5* [E. coli] (15) | [127] |
| Bamboo | |||||
| [Ag] | <100 | NPs grafted with acrylic acid | 98.7 [S. aureus] 100 [E. coli] | 84.9 [S. aureus] (50) 96.2 [E. coli] (50) | [128] |
| [CuO] | <100 [1 wt.%] | NPs grafted with acrylamide | 100 [S. aureus] 99 [E. coli] | 75 [S. aureus] (50) 75 [E. coli] (50) | [94] |
| [ZnO] | 10 [14 μg g−1] | NPs grafted with HSDA | 99.1 [S. aureus] 99.9 [E. coli] | 99 [S. aureus] (20) 98.9 [E. coli] (20) | [87] |
| Cellulose acetate | |||||
| [Ag] | 3–16 [0.05 wt.%] | Electrospinned nanofibers containing NPs | 99.9 [S. aureus] 99.9 [E. coli] 99.9 [K. pneumonia] 99.9 [P. aeruginosa] | n.a. | [104] |
| 21 [0.5 wt.%] | Electrospinned nanofibers containing NPs | 99.9 [S. aureus] 99.9 [E. coli] 99.9 [K. pneumonia] 99.9 [P. aeruginosa] | n.a. | [129] | |
| Cotton | |||||
| [Alginate/TSA] | 99 [70 μg g−1] | Colloid NPs impregnated on fabric | 99.9 [E. coli] 99.9 [S. aureus] | 99 [E. coli] (30) | [130] |
| [Ag] | 0.65 [7 wt.%] | Colloid NPs padded on fabric | 1.21* [S. aureus] | 0 [S. aureus] (5) | [23] |
| 1–2 [0.8 wt.%] | Silica–silver core–shell particle deposited by Pad-dry-cure method | <1* [E. coli] | n.a. | [40] | |
| 1.6 [10 μg g−1] | Biosynthesized NPs impregnated on cotton | 99 [S. aureus] | n.a. | [131] | |
| 2–5 [3.17 wt.%] | Colloid NPs synthetized and adsorbed on fabric | 99.9 [S. typhimurium] 97 [S. aureus] | 96 [S. typhimurium] (30) 93 [S. aureus] (30) | [98] | |
| 2–12 [1215 μg g−1] | Colloid NPs adsorbed by exhaustion method | 6–7* [E. coli] | n.a. | [39] | |
| 2–12 [385 μg g−1] | Colloid NPs adsorbed by exhaustion method | 4–5* [E. coli] | n.a. | [39] | |
| 2–5 [30 μg g−1] | Colloid NPs padded on fabric | 99.9 [S. aureus] 99.9 [K. pneumoniae] | 94.5 [K. pneumoniae] (20) 97.2 [S. aureus] (20) | [132] | |
| 2–6 | NPs−poly(acrylate) clusters impregnated on fabric | >1* [S. aureus] >1* [S. epidermidis] >1* [P. aeruginosa] >1* [C. albicans] | n.a. | [44] | |
| 2–8 [0.7 wt.%] | Cellulose–Gum polymer–Ag nanocomposite adsorbed by exhaustion method | >1.7* [E. coli] | n.a. | [49] | |
| 3 [20 μg g−1] | Colloid NPs padded on fabric | 99.9 [S. aureus] 99.9 [K. pneumoniae] | 99.9 (10) | [25] | |
| 3–20 [336 μg g−1] | Colloid NPs/PEG adsorbed by exhaustion method | 10.5* [E. coli] 6.7* [S. aureus] | 1* [E. coli] (50) 1.8* [S. aureus] (50) | [133] | |
| 3–20 [336 μg g−1] | Colloid NPs adsorbed by exhaustion method | 2–3* [E. coli] | n.a. | [39] | |
| 3–20 [894 μg g−1] | Colloid NPs adsorbed by exhaustion method | 5–6* [E. coli] | n.a. | [39] | |
| 3–8 [108 μg g−1] | Biosynthesized NPs padded on fabric | 96 [E. coli] 98 [S. aureus] | 55 [E. coli] (20) 59 [S. aureus] (20) | [24] | |
| 5 | Dodecanethiol-capped NPs in silica sol | 40 [E. coli] | n.a. | [42] | |
| 6–8 [100 μg g−1] | Pad-dry-curePad-dry-cure method | 96 [E. coli] 98.3 [S. aureus] | 59 [E. coli] (20) 62 [S. aureus] (20) | [48] | |
| 6–8 [50 μg g−1] | Pad-dry-cure method | 96 [E. coli] 96.4 [S. aureus] | 56.6 [E. coli] (20) 60.9 [S. aureus] (20) | [48] | |
| 7–11 [758 μg g−1] | Microwave synthetized colloid NPs padded on fabric | 99.9 [E. coli] 99.9 [S. aureus] | 37 [E. coli] (15) 26 [S. aureus] (15) | [134] | |
| 8 | Pad-dry-cure method | 99.9 [E. coli] 99.9 [S. aureus] | 99.9 [E. coli] (30) 99.9 [S. aureus] (20) | [135] | |
| 10 | NPs with dendrimers in Pad-dry-cure method | 95 [E. coli] 95 [S. aureus] | n.a. | [50] | |
| 10 [50 μg g−1] | Colloid NPs impregnated on fabric | 99.9 [E. coli]99.9 [S. aureus] 99.9 [C. albicans] | 99.9 (5) | [136] | |
| 10–20 | Colloid NPs impregnated by US | 63.6 [B. linens] 62.7 [S. epidermidis] | n.a. | [137] | |
| 10–110 [8.2 μg g−1] | Spherical AgNPs deposition by US | 99.9 [S. aureus] 99.9 [E. coli] | 0 [S. aureus] (5) 0 [E. coli] (5) | [34] | |
| 11 | NPs grafted with HBP-NH2 | 99.4 [S. aureus] 99.4 [E. coli] | 96.7 [S. aureus] (50) 96.5 [E. coli] (50) | [138] | |
| 15–30 | NPs adsorbed by exhaustion method | 20 [F. oxysporum] 25 [A. brassicicola] | n.a. | [139] | |
| 18 [88 μg g−1] | Colloid NPs with HBP-NH2 impregnated on fabric | 99 [E. coli] 99.3 [S. aureus] | 98.8 [E. coli] (20) 99 [S. aureus] (20) | [45] | |
| 20 | Covalent bond of AgNPs polystyrene-block-polyacrylic acid reverse micelle cores | >20* [E. coli] >1* [S. aureus] | 0* [E. coli] (5) >1* [S. aureus] (20) | [140] | |
| 20 [2 wt.%] | Colloid NPs impregnated on fabric | >1.5* [E. coli] | n.a. | [141] | |
| 20–110 [14.1 μg g−1] | Disc AgNPs deposition by US | 99.9 [S. aureus] 99.9 [E. coli] | 0 [S. aureus] (5) 0 [E. coli] (5) | [34] | |
| 20–60 [1500 μg g−1] | UV-assisted Pad-dry-cure method | >1* [E. coli] >1.5* [S. aureus] | <1* [E. coli] (10) >1.5* [S. aureus] (10) | [58] | |
| 30 [54 μg g−1] | Colloid NPs adsorbed by exhaustion in CF4-plasma treated fabric | 77 [P. aeruginosa] 68 [E. faecalis] | n.a. | [60] | |
| 30–50 | Colloid NPs adsorbed by exhaustion method | 99.9 [E. coli] 99.8 [S.epidermis] | 93.3 [E. coli] (20) 90.8 [S. epidermis] (20) | [142] | |
| 30–200 [140 μg g−1] | Silica/AgNPs Pad-dry-cure method | 100 [E. coli] 100 [S. aureus] 100 [A. niger] | n.a. | [41] | |
| 32–64 [12.8 μg g−1] | Prism AgNPs deposition by US | 99.9 [S. aureus] 99.9 [E. coli] | 12.5 [S. aureus] (5) 49.9 [E. coli] (5) | [34] | |
| 35–80 [0.5 wt.%] | Colloid NPs deposition by UV | 5* [E. coli] 4* [S. aureus] 5* [C. albicans] 3* [P. p43] | n.a. | [143] | |
| 41 [5300 μg g−1] | Ethanolic solution of AgNO3 and butylamine impregnated on fabric | 98 [E. coli] 95 [S. aureus] | n.a. | [43] | |
| 50 [30 wt.%] | Gas-phase reaction between phosphine and copper sulphate and AgNO3 | 100 [S. aureus] | 100 [S. aureus] (10) | [144] | |
| 50 [9.4 μg g−1] | Polygonal AgNPs deposition by US | 99.9 [S. aureus] 99.9 [E. coli] | 25 [S. aureus] (5) 0 [E. coli] (5) | [34] | |
| 60 [100 μg g−1] | NPs and BTCA impregnated on fabric | 100 [E. coli] 100 [S. aureus] | 96 [E. coli] 92 [S. aureus] (30) | [46] | |
| 75 | Microfibers containing NPs by UV irradiation | 80 [E. coli] | n.a. | [57] | |
| 80 [26 μg g−1] | Colloid NPs adsorbed by exhaustion in CF4-plasma treated fabric | <60 [P. aeruginosa] <60 [E. faecalis] | n.a. | [60] | |
| 80 [6 wt.%] | Synthesis and deposition of NPs using US irradiation | 100 [E. coli] 100 [S. aureus] | n.a. | [54] | |
| 180 [4 wt.%] | Colloid NPs padded on fabric | 1.66* [S. aureus] | 4.2* (5) | [23] | |
| 200 [350 μg g−1] | Colloid NPs impregnated on fabric | 99 [E. coli] 100 [S. aureus] 100 [P. aeruginosa] | n.a. | [145] | |
| 200 [452 μg g−1] | Colloid NPs padded on fabric | 99.9 [E. coli] 99.8 [S. aureus] | n.a. | [146] | |
| >200 [16.8 μg g−1] | Hierarchical AgNPs deposition by US | 99.9 [S. aureus] 99.9 [E. coli] | 91.3 [S. aureus] (5) 99.9 [E. coli] (5) | [34] | |
| 257 [34.5 wt.%] | NPs impregnated on fabric | 99.9 [E. coli] | 199.9 [E. coli] (10) | [147] | |
| [Ag/Chitosan] | 40 [1 wt.%] | Pad-dry-cure method | 31* [E. coli] 26* [S. aureus] | 15* [E. coli] (20) 17* [S. aureus] (20) | [148] |
| 50–175 | Colloid NPs impregnated on fabric | 3* [E. coli] | n.a. | [52] | |
| [Ag/Chitosan/TiO2 ] | 5000 [7 wt.% ] | Pad-dry-cure method | 98 [E. coli] 100 [S. aureus] | n.a. | [149] |
| [Chitosan] | 5–180 [0.5 wt.%] | Colloid NPs impregnated on fabric | 99.9 [E. coli] 99.9 [S. aureus] | 65 [E. coli] (20) 78 [S. aureus] (20) | [124] |
| 40 | Colloid NPs impregnated on fabric by US | 5 [E. coli] 25 [E. faecalis] | n.a. | [123] | |
| 350 [0.8 wt.%] | NPs grafted with GPTMS | 80 [E. coli] 80 [M. lutues] | n.a. | [125] | |
| [Chitosan/Alginate] | 35 | Pad-dry-cure method of NPs loaded with leaf extract | 100 [B. cereus] 98 [E. coli] 100 [P. aeruginosa] 100 [S. aureus] | 95 [B. cereus] (30) 87 [E. coli] (30) 98 [P. aeruginosa] (30) 98 [S. aureus] (30) | [150] |
| [CuO] | 10 [5 wt.%] | Colloid NPs impregnated on fabric by US | 99.9 [E. coli] 99.9 [S. aureus] | n.a. | [56] |
| 10–20 [1.5 wt.%] | Colloid NPs impregnated on fabric by US | 99.8 [E. coli] | n.a. | [55] | |
| 15 [1.4 wt.%] | Colloid NPs impregnated on fabric by US | 99.9 [E. coli] 99.9 [S. aureus] | n.a. | [91] | |
| 40–60 [0.2 wt.%] | Pad-dry-cure method | 93.7 [E. coli] 95 [S. aureus] | 48 [E. coli] (15) 45 [S. aureus] (15) | [151] | |
| 50 | Microencapsulated NPs adsorbed by exhaustion method | 92.71 [E. coli] 100 [S. aureus] | 86 [E. coli] (10) 92 [S. aureus] (10) | [38] | |
| 60–75 [2 wt.%] | Pad-dry-cure method | 86.5 [E. coli] 94.2 [S. aureus] | 9.8 [E. coli] (20) 12 [S. aureus] (20) | [92] | |
| 60–80 [0.7 wt.%] | Colloid NPs impregnated on fabric by US | 73 [E. coli] 66 [S. aureus] 72 [MRSA] 50 [A. baumannii] 74 [P. aeruginosa] | 5 [E. coli] (65) 46 [S. aureus] (65) | [95] | |
| 100–150 | Colloid NPs coated by pad-dry-cure method | 80 [E. coli] 99 [S. aureus] 98 [A.niger] | n.a. | [93] | |
| 200–400 [0.74 wt.%] | Colloid NPs impregnated on fabric by US | 38 [E. coli] 38 [S. aureus] 52 [K. pneumonia] 52 [MRSA] 1 [A. baumannii] 15 [P. aeruginosa] | n.a. | [152] | |
| [TiO2 ] | 7 [2 wt.%] | Pad-dry-cure method | 72.9 [S. aureus] 74.5 [K. pneumonia] | 29.9 [S. aureus] (20) 30.5 [K. pneumonia] (20) | [153] |
| 10–15 [6 wt.%] | Colloid anatase NPs impregnated on fabric by US | 25 [E. coli] 94.4 [S. aureus] 59.5 [C. albicans] | n.a. | [154] | |
| 10–15 [8 wt.%] | Colloid rutile NPs impregnated on fabric by US | 6.9 [E. coli] 72.4 [S. aureus] 40.3 [C. albicans] | n.a. | [154] | |
| 10–15 [6 wt.%] | Colloid anatase NPs impregnated on fabric by US + UV | 29.2 [E. coli] 99.9 [S. aureus] 70.1 [C. albicans] | n.a. | [154] | |
| 10–15 [8 wt.%] | Colloid rutile NPs impregnated on fabric by US + UV | 31.9 [E. coli] 99.3 [S. aureus] 62.4 [C. albicans] | n.a. | [154] | |
| 12m [2 wt.%] | Pad-dry-cure method | 75.8 [S. aureus] 77.6 [K. pneumonia] | 34.7 [S. aureus] (20) 34.7 [K. pneumonia] (20) | [153] | |
| 13–20 [1 μg g−1] | Pad-dry-cure method | 94 [E. coli] 99 [S. aureus] | 83 [E. coli] (10) 86 [S. aureus] (10) | [76] | |
| <50 [5 wt.%] | Colloid NPs impregnated on fabric | 98 [S. aureus] 98 [K. pneumoniae] | n.a. | [75] | |
| 70–390 [0.5 wt.%] | Apatite-coated NPs by pad-dry-cure method | 5.5 [E. coli] 13.4 [S. aureus] 24.2 [M. luteus] | n.a. | [74] | |
| [ZnO] | 10–20 [0.8 wt.%] | Colloid NPs impregnated on fabric by US | 17 [E. coli] | n.a. | [55] |
| 20–100 | Colloid NPs impregnated on fabric by US | 100 [S. aureus] | n.a. | [155] | |
| 25 | ZnO nanoparticle incorporated PS-b-PAA coating | >1* [S. aureus] >1* [E. coli] | n.a. | [156] | |
| 30–40 [0.66 wt.%] | Roll to roll US coating on enzyme pre-treated fabric | 36 [S. aureus] 35 [P. aeruginosa] 25 [A. baumannii] 70 [E. coli] 31 [MRSA] | 15 [S. aureus] (10) 30 [P. aeruginosa] (10) 12 [A. baumannii] (10) 30 [E. coli] (10) 32 [MRSA] (10) | [157] | |
| 30–60 [10 wt.%] | Pad-dry-cure method | 99 [E. coli] 98 [M. luteus] | n.a. | [158] | |
| 30 [0.75 wt.%] | Colloid NPs deposition by US | 99.9 [E. coli] 99.9 [S. aureus] | n.a. | [83] | |
| 37 [20 wt.%] | Pad-dry-cure method | 100 [E. coli] 100 [S. aureus] | n.a. | [159] | |
| 38 [0.2 wt.%] | Pad-dry-cure method | 91.8 [S. aureus] 15 [K. pneumoniae] | n.a. | [84] | |
| 38 [0.6 wt.%] | Pad-dry-cure method | 96.8 [S. aureus] 99.9 [K. pneumoniae] | n.a. | [84] | |
| 38 [1 wt.%] | Pad-dry-cure method | 99.9 [S. aureus] 99.9 [K. pneumoniae] | n.a. | [84] | |
| <50 | Colloid NPs impregnated on fabric | 97 [S. aureus] 98 [K. pneumoniae] | n.a. | [75] | |
| 60–70 [0.75 wt.%] | Roll to roll US coating on fabric | 36 [S. aureus] 18 [P. aeruginosa] 11 [A. baumannii] 65 [E. coli] 38 [MRSA] | 15 [S. aureus] (10) 12 [P. aeruginosa] (10) 0 [A. baumannii] (10) 5 [E. coli] (10) 10 [MRSA] (10) | [157] | |
| <100 | Layer-by-layer deposition of NPs | 1.3* [S. aureus] | 0 [S. aureus] (20) | [85] | |
| 80–150 [4.3 wt.%] | Nanorods and chalcone solution padded on fabric | 99.9 [E. coli] 99.6 [S. aureus] 99.9 [P. aeruginosa] | n.a. | [36] | |
| 200 [2 wt.%] | Pad-dry-cure method | 80 [E. coli] 99.6 [S. aureus] | 75 [E. coli] (5) 98 [S. aureus] (5) | [160] | |
| [ZnO/Chitosan] | 15–60 [0.3 wt.%] | Colloid NPs impregnated on fabric by US | 99.9 [E. coli] 98.5 [S. aureus] | 85 [E. coli] (10) 70 [S. aureus] (10) | [161] |
| 28–100 [6 wt.%] | Pad-dry-cure method | 22* [E. coli] 25* [S. aureus] | n.a. | [89] | |
| 30 | Pad-dry-cure method | 100 [E. coli] 100 [M. luteus] | n.a. | [162] | |
| 60 [0.5 wt.%] | Colloid NPs impregnated on fabric by US | 40 [E. coli] 48 [E. faecalis] | n.a. | [123] | |
| [ZrO] | 2–5 [2.41 wt.%] | Colloid NPs synthetized and adsorbed on fabric | 98 [S. typhimurium] 95 [S. aureus] | 92 [S. typhimurium] (30) 90 [S. aureus] (30) | [98] |
| Cotton/polyester | |||||
| [Ag] | 30–200 [59 μg g−1] | Silica/AgNPs Pad-dry-cure method (65/35) | 100 [E. coli] 100 [S. aureus] 100 [A. niger] | n.a. | [41] |
| 100 [1000 μg g−1] | Colloid NPs impregnated on fabric (60/40) | 100 [S. aureus] | 100 [S. aureus] (4) | [163] | |
| [TiO2 ] | <50 [5 wt.%] | Colloid NPs impregnated on fabric (55/45) | 98 [S. aureus] 99 [K. pneumoniae] | n.a. | [75] |
| [ZnO] | 30 | Pad-dry-cure method | 100 [E. coli] 100 [M. luteus] | n.a. | [162] |
| 30–60 [10 wt.%] | Pad-dry-cure method (65/35) | 98 [E. coli] 99 [M. luteus] | n.a. | [158] | |
| <50 | Colloid NPs impregnated on fabric (55/45) | 98 [S. aureus] 99 [K. pneumoniae] | n.a. | [75] | |
| Polyacrylonitrile | |||||
| [Chitosan] | 1000 [15 wt.%] | Electrospinned nanofibers containing Chitosan | 100 [E. coli] 100 [S. aureus] 99.8 [P. aeruginosa] 100 [M. luteus] | n.a. | [122] |
| Polyamide | |||||
| [Ag] | 8 | Electrospinned nanofibers containing NPs | 99.9 [E. coli] | n.a. | [105] |
| 10 [4.46 μg g−1] | Colloid NPs impregnated on corona-plasma treated fabric | 99.9 [C. albicans] | 64.7 [C. albicans] (5) | [70] | |
| 10 [4.46 μg g−1] | Colloid NPs impregnated on corona-plasma treated fabric | 99.9 [E. coli] 99.9 [S. aureus] | 83.2 [E. coli] (5) 85.3 [S. aureus] (5) | [63] | |
| 10 [4.46 μg g−1] | Colloid NPs impregnated on corona-plasma treated dyed fabric | 98.6 [C. albicans] | n.a. | [64] | |
| 10 [4.46 μg g−1] | Colloid NPs impregnated on corona-plasma treated dyed fabric | 99.9 [E. coli] 99.9 [S. aureus] | n.a. | [164] | |
| 10–20 [0.025 wt.%] | Thermal reduction of silver acetate during melt processing of PA | 80.6 [E. coli] | n.a. | [101] | |
| 10–20 [0.06 wt.%] | Thermal reduction of silver acetate during melt processing of PA | 99.9 [E. coli] | n.a. | [101] | |
| 30–200 [31 μg g−1] | Silica/AgNPs Pad-dry-cure method | 100 [E. coli] 100 [S. aureus] 100 [A. niger] | n.a. | [41] | |
| 50–100 [1 wt.%] | Colloid NPs impregnated on fabric by US | 99 [P. aeruginosa] 99 [S. aureus] | n.a. | [66] | |
| 70 | Colloid NPs impregnated on fabric | 5–6* [E. coli] | n.a. | [165] | |
| 80 | Colloid NPs impregnated on dyed fabric | 100 [E. coli] 100 [S. aureus] 100 [P. aeruginosa] | 0 [E. coli] 0 [S. aureus] 0 [P. aeruginosa] | [166] | |
| <100 | Layer-by-layer deposition of NPs | 53 [S. aureus] | n.a. | [167] | |
| [CuO] | 85 [8.5 wt.%] | In situ produced NPs grafted with CTAB | >1* [S. aureus] | n.a. | [97] |
| [TiO2 ] | 21 [1 wt.%] | Electrospinned nanofibers containing NPs | 99 [E. coli] | n.a. | [73] |
| [ZnO] | <100 [5 wt.%] | Sheath-core fibers prepared by melt-spinning method | 100 [S. aureus] 100 [K. pneumoniae] | n.a. | [86] |
| <100 [1 wt.%] | Sheath-core fibers prepared by melt-spinning method | 95 [S. aureus] 68 [K. pneumoniae] | n.a. | [86] | |
| Poly(ε-caprolactone) | |||||
| [Ag-Zr(HPO4 )2 ] | 63.7 [1 wt.%] | Electrospinned nanofibers containing NPs | 98.4 [E. coli] 99.3 [S. aureus] | n.a. | [106] |
| Polyester | |||||
| [Ag] | 2–5 | Colloid NPs padded on fabric | 99.7 [S. aureus] 99.8 [K. pneumoniae] | 15.3 [K. pneumoniae] (20) 84.3 [S. aureus] (20) | [132] |
| 2–6 | Silver−poly(acrylate) NPs clusters impregnated on fabric | >1* [S. aureus] >1* [S. epidermidis] >1* [P. aeruginosa] >1* [C. albicans] | n.a. | [44] | |
| 3 [10 μg g−1] | Colloid NPs padded on fabric | 99.9 [S. aureus] 99.9 [K. pneumoniae] | n.a. | [25] | |
| 10 | NPs with dendrimers in Pad-dry-cure method | 95 [E. coli] 60 [S. aureus] | n.a. | [50] | |
| 10 [8.61 μg g−1] | Colloid NPs impregnated on corona-plasma treated fabric | 99.1 [C. albicans] | 96.7 [C. albicans] (5) | [70] | |
| 10 [8.61 μg g−1] | Colloid NPs impregnated on corona-plasma treated fabric | 99.9 [E. coli] 99.8 [S. aureus] | 99.9 [E. coli] (5) 99.6 [S. aureus] (5) | [63] | |
| 10 [8.61 μg g−1] | Colloid NPs impregnated on corona-plasma treated dyed fabric | 99.9 [C. albicans] | n.a. | [64] | |
| 10 [8.61 μg g−1] | Colloid NPs impregnated on corona-plasma treated dyed fabric | 99.9 [E. coli] 99.9 [S. aureus] | n.a. | [164] | |
| 11 [1 wt.%] | NPs plasma grafted with acrylic acid | 99.9 [E. coli] 99.9 [S. aureus] | n.a. | [168] | |
| <20 [10 μg g−1] | Colloid NPs impregnated on fabric | 100 [S. aureus] 42 [K. pneumoniae] | n.a. | [169] | |
| <20 [100 μg g−1] | Colloid NPs impregnated on fabric | 100 [S. aureus] 100 [K. pneumoniae] | n.a. | [169] | |
| 30–200 [8.9 μg g−1] | Silica/AgNPs Pad-dry-cure method | 100 [E. coli] 100 [S. aureus] 100 [A. niger] | n.a. | [41] | |
| 40–70 [155 μg g−1] | Colloid NPs impregnated on RF-plasma treated fabric | 99.9 [E. coli] 99.9 [S. aureus] | 99.9 [E. coli] 5) 99.9 [S. aureus] 5) | [61] | |
| 80 [93 μg g−1] | Colloid NPs impregnated on corona-plasma treated fabric | 19 [E. coli] 67 [S. aureus] 74 [S. faecalis] 6 [P. aeruginosa] | n.a. | [62] | |
| [Ag/Chitosan] | 166 [0.2 wt.%] | Colloid NPs impregnated on PVP treated fabric | 100 [S. aureus] | n.a. | [53] |
| [Chitosan] | 5–180 [0.5 wt.%] | Colloid NPs impregnated on fabric | 90 [E. coli] 99.9 [S. aureus] | 50 [E. coli] (20) 75 [S. aureus] (20) | [124] |
| 115 [0.2 wt.%] | Colloid NPs impregnated on PVP treated fabric | 90 [S. aureus] | n.a. | [53] | |
| [Fe3 O4 ] | 30–40 | In situ synthesis of NPs | 100 [S. aureus] | n.a. | [100] |
| [α-Fe2 O3 ] | 40–50 | In situ synthesis of NPs | 80.5 [S. aureus] | n.a. | [100] |
| [SiO2 /Ag/CuO] | 500/30/17 [2.5 wt.%] | Top-coating with Pericoat PU 340 NEW paste containing NPs | 100 [E. coli] 99.8 [S. aureus] 99.9 [K. pneumoniae] 100 [C. albicans] 99.4 [A. niger] 96.5 [T. mentagraphytes] | 99.9 [E. coli] (20) 99.8 [S. aureus] (20) 99.9 [K. pneumoniae] (20) 100 [C. albicans] (20) 56.4 [A. niger] (20) 92.1 [T. mentagraphytes] (20) | [170] |
| [TiO2 ] | 6 [2.1 wt.%] | Alginates and colloid NPs impregnated on fabric | 99.9 [E. coli] | 99.8 (5) | [171] |
| Polyethylene | |||||
| [Ag/Chitosan] | 5 [1.1 wt.%] | Electrospinned nanofibers containing NPs | 99.9 [E. coli] | n.a. | [107] |
| Polyethylene/Chitosan | |||||
| [Ag] | 12–18 [1.3 wt.%] | Electrospinned nanofibers containing NPs | 15* [E. coli] 20* [S. aureus] 18* [P. aeruginosa] 12* [C. albicans] | n.a. | [172] |
| Polyethylene/polypropylene | |||||
| [Ag] | <10 [12 μg g−1] | Colloid NPs padded on non-woven fabric | 99.8 [S. aureus] 99.9 [K. pneumoniae] | n.a. | [173] |
| Poly(l-lactide) | |||||
| [Ag] | 30 [32 wt.%] | Electrospinned nanofibers containing NPs | 94.2 [E. coli] 98.5 [S. aureus] | n.a. | [108] |
| 35 [5 wt.%] | Electrospinned nanofibers containing NPs | 5* [E. coli] 5* [S. aureus] | n.a. | [109] | |
| Polypropylene | |||||
| [Ag] | 15 [0.3 wt.%] | Sheath-core fibers prepared by melt-spinning method | 99.9 [S. aureus] 99.9 [K. pneumoniae] | n.a. | [103] |
| 30 [0.1 wt.%] | Twin-screw mixer extrusion | 99.9 [S. aureus] | n.a. | [102] | |
| [TiO2 /Ag] | 60–100 [0.2 wt.%] | Sheath-core fibers prepared by melt-spinning method | 99.2 [S. aureus] | n.a. | [78] |
| Poly(vinyl alcohol) | |||||
| [Ag] | 6 [0.1 wt.%] | Electrospinned nanofibers containing NPs | 99.9 [S. aureus] 99.9 [K. pneumoniae] | n.a. | [110] |
| [Ag/Chitosan] | 2–10 [1 wt.%] | Electrospinned nanofibers containing NPs | 99.9 [E. coli] | n.a. | [111] |
| 20 [0.6 wt.%] | Electrospinned PVA nanofibers containing NPs | 100 [E. coli] | n.a. | [51] | |
| [TiO2 /Ag/Chitosan] | 100 [0.04 wt.%] | Electrospinned nanofibers containing NPs | 99 [E. coli] 98 [S. aureus] | n.a. | [79] |
| Poly(vinyl alcohol)/Silk | |||||
| [Ag] | 3.8 | Electrospinned nanofibers containing NPs | >1* [E. coli] >1* [S. aureus] | n.a. | [112] |
| Silk | |||||
| [Ag] | 4.3 [116.5 μg g−1] | Colloid NPs and PNP impregnated on fabric | 99.5 [E. coli] 99.9 [S. aureus] | 98.9 [E. coli] (30) 99.4 [S. aureus] (30) | [174] |
| 5–50 [2.3 wt.%] | Colloid NPs impregnated on fabric | 99.9 [S. aureus] | n.a. | [175] | |
| 15–30 | Colloid NPs adsorbed by exhaustion method | 45 [F. oxysporum] 50 [A. brassicola] | n.a. | [139] | |
| 10 [40 μg g−1] | Colloid NPs applied by exhaustion | 100 [S. aureus] | 84 [S. aureus] (10) | [176] | |
| <10 | UV-assisted in situ synthesis of AgNPs | 1.59* [E. coli] 1.84* [S. aureus] | n.a. | [65] | |
| <10 [50 μg g−1] | Colloid NPs applied by exhaustion in US bath | 94 [E. coli] 100 [S. aureus] | n.a. | [177] | |
| 11.5 [13 μg g−1] | Colloid NPs impregnated on fabric | >1* [M. lysodeikticus] >1* [E. coli] >1* [S. aureus] | n.a. | [178] | |
| <20 | Synthesis of NPs on silk fibers via γ-ray irradiation | 96 [S. aureus] | 85 [S. aureus] (10) | [179] | |
| 30–200 [170 μg g−1] | Silica/AgNPs Pad-dry-cure method | 100 [E. coli] 100 [S. aureus] 100 [A. niger] | n.a. | [41] | |
| 35 [60 μg g−1] | Colloid NPs applied by exhaustion | 100 [S. aureus] | 78 [S. aureus] (10) | [176] | |
| 50 [268.6 μg g−1] | Colloid NPs impregnated on fabric | 99 [E. coli] | 98 [E. coli] (50) | [180] | |
| <100 [98.7 μg g−1] | Multi-amidine/silver nitrate sol added by steam method | 99.9 [E. coli] 99.5 [S. aureus] | >97.4 [E. coli] (50) | [181] | |
| <100 | Colloid NPs impregnated on fabric | 100 [E. coli] | n.a. | [182] | |
| <100 | Layer-by-layer deposition of NPs | 80 [S. aureus] | n.a. | [167] | |
| [Au] | 21 [0.21 wt.%] | In situ synthesized NPs impregnated on fabric | 100 [E. coli] | n.a. | [99] |
| [Chitosan] | 20.8 [1 wt.%] | Colloid NPs impregnated on fabric | 95 [S. aureus] | 90 [S. aureus] (20) | [183] |
| [TiO2 ] | 50 [25 μg g−1] | Colloid NPs and PUA applied by dyeing | 19* [E. coli] 23* [S. aureus] | n.a. | [184] |
| [TiO2 /Ag] | 20/5 [1 wt.%] | DHPBA modified NPs padded on fabric | >1* [E. coli] >1* [S. aureus] >1* [P. aeruginosa] | n.a. | [80] |
| Viscose | |||||
| [Ag] | 2–9 [4 wt.%] | AgNPs SiO2 Sol-gel coating | 53 [A. niger] 41 [B. subtilis] 88 [P. putida] | n.a. | [185] |
| 30–200 [230 μg g−1] | Silica/AgNPs Pad-dry-cure method | 100 [E. coli] 100 [S. aureus] 100 [A. niger] | n.a. | [41] | |
| [Chitosan] | 5–180 [0.5 wt.%] | Colloid NPs impregnated on fabric | 99.9 [E. coli] 99.9 [S. aureus] | 55 [E. coli] (20) 76 [S. aureus] (20) | [124] |
| Wool | |||||
| [Ag] | 1–7 | Photocured Carboxymethyl Starch NPs | 24* [S. aureus] 22* [E. coli] | 3* [S. aureus] (15) 1* [E. coli] (15) | [127] |
| 2–6 | Silver−poly(acrylate) NPs clusters impregnated on fabric | >1* [C. albicans] | n.a. | [44] | |
| 4.2 [5 μg g−1] | Colloid silver NPs impregnated by pad-dry-cure | 99.9 [S. aureus] 99.7 [K. pneumoniae] | n.a. | [186] | |
| 15 × 6 | Nanodisc colloid NPs impregnated on fabric | 98.5 [E. coli] | n.a. | [35] | |
| 22 × 14 | Nanodisc colloid NPs impregnated on fabric | 93.8 [E. coli] | n.a. | [35] | |
| 45–60 | Colloid NPs adsorbed by exhaustion method | 99.9 [E. coli] 98.9 [S. epidermis] | 92 [E. coli] (20) 91.7 [S. epidermis] (20) | [142] | |
| 48 × 5 | Nanoprism colloid NPs impregnated on fabric | 77 [E. coli] | n.a. | [35] | |
| 30–200 [310 μg g−1] | Silica/AgNPs Pad-dry-cure method | 18 [E. coli] 56 [S. aureus] 50 [A.niger] | n.a. | [41] | |
| [SiO2 /Ag] | 34.6 | Colloid NPs impregnated on fabric | 71 [E. coli] | n.a. | [187] |
| 60 [4.3 wt.%] | Colloid NPs impregnated on RF-plasma treated fabric | 85 [E. coli] 95 [S. aureus] | 70 [E. coli] (20) 73 [S. aureus] (20) | [71] | |
| [TiO2 /Ag] | 21 [1 wt.%] | TiO2 NPs in silver nitrate solution impregnated on fabric | 100 [E. coli] 100 [S. aureus] | n.a. | [81] |
| Wool/polyester | |||||
| [Ag] | 30–200 [250 μg g−1] | Silica/AgNPs Pad-dry-cure method (45/55) | 100 [E. coli] 100 [S. aureus] 0 [A.niger] | n.a. | [41] |
| [TiO2 ] | 21 [0.25 wt.%] | Colloid NPs crosslinked with BTCA on fabric (45/55) | 99 [E. coli] | n.a. | [188] |
| 21 [0.75 wt.%] | Colloid NPs crosslinked with BTCA on fabric (45/55) | 100 [E. coli] | n.a. | [188] |
*Inhibition zone in mm; n.a. Not available.
Currently, nanotechnology is considered the most promising technology for novel textile commercial applications since it allows the permanent and effective functionalization of substrates without affecting their macro-scale properties, such as breathability or hand feel [18]. Nanoparticles as antimicrobial agents have increasingly been used in textile research due to their unique physico-chemical properties and biological activity, which may differ significantly from ion and bulk materials [21]. However, nanoparticles (NPs) in humans may affect normal cellular proliferation and protein functions primarily due to their metallic nature, and the generation of reactive oxygen species may initiate pro-inflammatory and toxic activities [22]. The first chapter of the manuscript is dedicated to AgNPs because most of the research in this field was conducted using this metal. For the same reason, because the majority of the information regarding the effect of NP morphology and deposition methods was only studied on silver, three subchapters (NP morphology, chemical deposition methods and physical deposition methods) were added. The first subchapter is dedicated to the importance of size, shape, composition, crystallinity and structure of NPs on their antimicrobial activity. The second is dedicated to chemical deposition methods, with special emphasis on the new routes for the deposition of NPs based on environmental benign natural polymers. The third subchapter is about physical deposition methods, focusing on plasma technology. The following chapters report on research using other antimicrobial metal and metal oxide NPs and nanofibers. The last chapter reports on the recent use of natural polymer NPs, especially chitosan, in the antimicrobial finishing of textiles due to the environmental and toxicological concerns regarding the use of heavy metals for the production of NPs. Thus, due to the increasing dichotomy between environmental and health concerns and the potential benefits of using NPs as finishing agents, this review offer the opportunity to update and critically discuss the latest advances and applications for the textile industry.
Nanosilver
As we can see in table 1, several methods have been used for surface nanomodifications of textiles, but most of the research has been performed using nanosilver immobilized on cotton, polyester, polyamide, silk and wool fabrics by conventional dip- or pad-dry methods [23–25]. The term 'nanosilver' is conventionally attributed to silver metals, but a fraction of the silver salts could also fall under the NP definition according to the International Standard Organisation (ISO), which defines an NP as having a maximum diameter of 100 nm in at least three dimensions. However, all particles with a diameter between 100 to 1000 nm were also assumed to contain NPs, unless there was concrete information about the size distribution and the stability of agglomerates. Taking this into account, according to Windler et al up to 80% of all silver used in textiles (45 metric tonnes) may be considered in the nanoform [26]. Considerable work has been done in the functionalization of textile materials with AgNPs. Most of the research is focused on the antimicrobial effects of modified textile materials, but no evident conclusions about the binding mechanism between AgNPs and textile fibers has been proposed. Thus, due to the huge amount of AgNP-containing textiles, some concerns have been raised about the release of silver into the environment after repeated washing [27, 28]. Some authors questioning the use of AgNPs of lower than 30 nm in textiles due to the additional effort it requires towards synthesis, stabilization and incorporation when the same results could be obtained simply by immersing the fabric in solutions of AgNO3 [29]. The critics are basing their questions on the difficulties in achieving small particle sizes of narrow size distributions with green processes and on the toxicity concerns of particles with a size between 1 and 10 nm, which can penetrate human skin [30]. Nowadays, the release of silver is an emerging environmental problem. Therefore, it is expected that further research will be more oriented towards the environmental, nanotechnological and regulatory aspects of the exploitation of textile products with deposited or immobilized NPs [31].
NP morphology
The way in which NPs alter surface properties and impart textile antimicrobial functions is mainly determined by their size, shape, composition, crystallinity and structure [32]. It has been described that AgNPs of between 1 and 10 nm present a greater impact on bacteria than larger particles, and that triangular-shaped NPs display greater biocidal action than rod- or spherical-shaped ones [33]. Recent results of studies on the antimicrobial effect on cotton fabric of different morphologies of AgNPs, such as spherical, polygonal, disk, prism, and hierarchical assemblies (figure 1), confirmed that non-spherical morphologies, such as polygonal-, prism- and hierarchical-like shapes, in comparison with spherical and disc morphologies exhibited a stronger growth-inhibitory effect against Gram-negative and Gram-positive bacteria. In addition, among various tested morphologies, the hierarchical-like morphology showed very good antimicrobial activity after five washing cycles [34]. On the other hand, in a few other works available in the literature using nano-rods, -discs and -prisms in textiles, moderate biocide activity is shown when compared with conventional spherical-shaped NPs [35, 36]. Supposedly, the significantly larger surface area of the NPs allows higher contact with bacteria, enhancing their bactericidal activity. However, other factors, such as dielectric and quantum confinement effects, could be responsible for the different properties of metal or metal-oxide NPs with respect to bulk materials [37].
Figure 1. Close-up of the TEM image of AgNPs (X200000 magnification) in different shapes and sizes. (1) Cubic, (2) Spherical ∼10 nm, (3) Triangular, (4) Spherical ∼60 nm, (5) Rod-like. TEM was performed using a JEOL JEM 1400 TEM (Tokyo, Japan) operating at an acceleration voltage of 120 kV. Nanoparticle sample was applied to glow-discharged carbon-coated copper grids followed by negative staining with a solution of 1% (w/v) uranyl acetate.
Download figure:
Standard image High-resolution imageChemical deposition methods
Most of the methods used in AgNP production are based on reactions in the liquid medium and often require environmentally hazardous surfactants, reducing agents and templates for the synthesis of AgNPs [38, 39]. Several studies reporting on silica–silver core–shell NPs mainly review the chemical synthesis processes and their characterization. Silica is a class of very important core materials for immobilizing NPs on its surface due to its high chemical and thermal stability, chemical inertness, large surface areas, and good compatibility with other materials. Nischala et al synthesized extremely small (1–2 nm) AgNPs attached on silica core particles with an average size of 270 nm using a simple one-pot chemical method. The silver containing silica core–shell particles immobilized on cotton did not leach out from the fabric and showed excellent antibacterial activity even after 10 washing cycles [40]. Novel fiber–silica–Ag composites with biocidal activity were also successfully produced by chemically modifying cotton, wool, silk, polyester and polyamide fabrics. The results show that the chemical and morphological structures of the fibers directly influenced their absorptivity and affinity for the AgNPs. On the other hand, a chemical strong-binding of Ag to the fibers seems to significantly reduce the effectiveness of the antimicrobial activity of the AgNPs [41]. Other methods include the reduction of silver ions by ethanol or isopropanol or the coating with acrylates or cross-linkable polysiloxanes to stabilize NP dispersion onto the fabric [32, 42–46]. The introduction of green chemistry into nanotechnology is one of the most important topics in nanoscience research today. The main purpose is to avoid the environmental toxicity or biological hazards normally associated with the preparation of AgNPs using synthetic reducing agents. To date, new routes for the development of NPs based on environmentally benign natural polymers such as chitosan, hyaluronan, starch, and cyclodextrin have been explored [47]. Hebeish et al synthesized small AgNPs using hydroxypropyl starch as both a reducing and stabilizing agent, retaining excellent antibacterial properties even after 20 washing cycles, reflecting the importance of binders in the fixation of AgNPs on the surface of the fabrics [48]. Raghavendra et al tested on cellulose fibers several natural carbohydrates such as gum acacia and gaur gum as an effective reducing agent for the green synthesis of AgNPs from AgNO3. The thermal stability and mechanical properties of the cellulosic composites were found to be better than cellulose fibers alone [49]. Mahltig et al fabricated hybrid nanomaterials based on dendrimers as polymeric stabilizers for the preparation of AgNPs used as finishing agents to produce antimicrobial textiles. The results confirmed that the antimicrobial effect rises with increases in the dendrimers' generations due to decreasing size of the formed AgNPs. By changing thermal fixation and dendrimers' generations, the strength of the antimicrobial effect can be controlled [50]. Several works involve chemical modification of textile fabrics by natural, biocompatible and biodegradable polysaccharide chitosan followed by incorporating AgNPs into the fabrics. Abdelgawad et al produced antibacterial nanofiber mats of PVA loaded with AgNPs enveloped in chitosan after reduction with glucose. The results showed superior properties and synergistic antibacterial effects by combining chitosan with AgNPs [51]. Other authors produced silver-loaded chitosan NPs attached to textiles, which also exhibited excellent antibacterial activity [52, 53].
Physical deposition methods
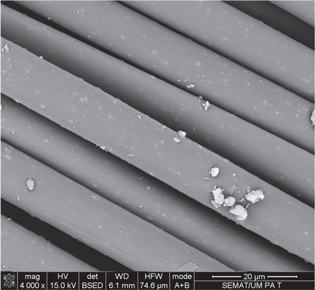
In the last decade, physical methods such as ultrasound [54–56], UV irradiation [57–59], plasma pre-treatment and ion-beam-assisted deposition [60–64] have been proved to be effective for the deposition, insertion and synthesis of well-dispersed nanophase materials on textiles. Lu et al developed a UV-assisted in situ synthesis approach to immobilize AgNPs on silk fibers for antibacterial applications. Results show that AgNPs with excellent crystalline structures are efficiently attached on the silk surface in an irradiation time-dependent manner [65]. Sonochemical reactions are capable of enhancing AgNP adhesion to the fabric surface by physical or chemical bonding depending on the nature of the substrate [66]. Pre-treatment of textiles by low-pressure plasmas can also improve loading of AgNPs from colloids (figure 2). Different plasma particles (e.g. electrons, ions, free radicals, photons) provide superficial functionalization and etching of the fiber without deterioration of bulk properties. Plasma is particularly important for the surface activation of hydrophobic synthetic fibers such as polyester and polyamide fabrics because it makes fibers more accessible to water and chemical species [67–69]. However, little research using plasma pre-treatment reports about antimicrobial activity on fabrics. The Serbian group headed by M Radetic reported great stability and uniform AgNPs coatings, as well as high antibacterial activity and laundering durability, using several plasma sources such as low-temperature air radio frequency (RF), dielectric barrier discharge (DBD) and corona discharge in different textile materials [61, 63, 64, 70]. Although RF-powered plasma devices allow easier control of properties and uniformity, this system requires more complex handling and a vacuum system, which can be avoided by using DBD and corona discharges at atmospheric pressure. Other groups working with plasma (including corona and CF4-plasma) have obtained similar results on AgNPs deposition, but with lower biocide performance [60, 62, 71].
Figure 2. SEM images (X4000 magnification) of antimicrobial AgNP aggregates deposited on DBD plasma pre-treated polyamide 6,6 fibers. Images were carried in a FEG-SEM, NOVA 200 Nano SEM, FEI Company. Secondary and backscattering electron images were performed with an acceleration voltage of 5 kV and 15 kV, respectively. Samples were covered with a film of Au–Pd (80–20 wt.%) in a high-resolution sputter coater, 208 h Cressington Company, coupled to a MTM-20 Cressington High Resolution Thickness Controller.
Download figure:
Standard image High-resolution imageNanotitanium dioxide
Nano TiO2, one of the most powerful photocatalytic materials, possesses high activity, strong oxidizing power and long-term stability [72]. When illuminated under UV light with wavelengths lower than 385 nm, nano TiO2 electrons are excited from the valence band to the conduction band [73]. The positive hole in the valence band can then react with water or hydroxide ions adsorbed on the surface to produce hydroxyl radicals, and the electron in the conduction band can reduce O2 to produce superoxide ions. These two highly reactive species are able to decompose a variety of organic materials, including microorganisms [74]. However, there are few reports on the use of TiO2 nanomaterial for textile applications, and only NPs with a diameter lower than 20 nm have shown effective, but not complete, antimicrobial activity in cotton, polyester, polyamide and wool/polyester fabrics [73–75].
Khurana et al observed that cationic as well as non-ionic dispersing agents led to a reduction in size of the TiO2 NPs produced by sol gel methods, whereas anionic dispersing agents led to an increase in particle size. The TiO2 NPs so synthesized were successfully applied onto cotton while maintaining their antimicrobial activity for up to 10 washes with the help of a binder [76]. Although there are numerous advantages in utilizing nano TiO2 in textiles, some drawbacks have also been reported. First, due to its high band gap, semiconductor TiO2 shows photocatalytic activity under UV rays, which practically limits the use of sunlight or visible light as an irradiation source. Second, the electron-hole recombination rate is too high, resulting in low photocatalytic efficiency. It has been suggested that through adding noble metals to the surface of TiO2, photocatalytic activity can be increased by extending the light absorption range of TiO2 from UV to the visible range [77]. Some examples using mixed silver/TiO2 are found in the existing literature for polypropylene, poly (vinyl alcohol), silk and wool, but they did not apparently show any additional advantages over silver [78–81]. Moreover, the dissipation mechanism of the UV energy is not often considered. A direct application of TiO2 to products such as paint, textile, plastics and paper can lead to the creation of free radicals with consequent photochemical decomposition of the substrates [74]. Free radicals are also implicated in a number of potential health issues such as skin aging. However, free radical generation can be reduced by over 90% by incorporating a dopant ion within the titanium oxide lattice structure [82].
Nanozinc oxide
ZnO NPs exhibit strong antibacterial activities on a broad spectrum of bacteria on cotton [36, 55, 75, 83–85], polyamide [86] and bamboo fabric [87]. Moreover, excellent multi-functional textiles with good UV protection in addition to very good antibacterial properties against Gram positive and Gram negative bacteria can be obtained using ZnO in combination with synthetic [88] and natural organic polymers such as chitosan [89]. The use of functional polymer matrices such as PMME or PNIPAM as a dispersion medium for ZnO NPs results in improved functional and bonding properties in fabrics (figure 3). Similar to TiO2, the photocatalytic generation of hydrogen peroxide was suggested to be one of the primary mechanisms. In addition, penetration of the cell envelope and disorganization of bacterial membrane upon contact with ZnO NPs were also indicated to inhibit bacterial growth. However, the role of the Zn2+ ion released from the dissolution of ZnO is not yet clear, and the antibacterial mechanism of ZnO is still under investigation [90].
Figure 3. SEM images (X1500 and X50000 magnification) of antimicrobial ZnO NPs—PNIPAM composite coated on DBD plasma pre-treated cotton fibers. Images were carried in a FEG-SEM, NOVA 200 Nano SEM, FEI Company. Secondary and backscattering electron images were performed with an acceleration voltage of 5 kV and 15 kV, respectively. Samples were covered with a film of Au–Pd (80–20 wt.%) in a high-resolution sputter coater, 208 h Cressington Company, coupled to a MTM-20 Cressington High Resolution Thickness Controller.
Download figure:
Standard image High-resolution imageNanocopper
Limited information, almost exclusively on cotton, is available on the antimicrobial activity and action mechanism of nano CuO in textiles [38, 55, 56, 91–93]. CuO is cheaper than silver, easily mixed with polymers and relatively stable in terms of both chemical and physical properties. Very recently, Teli et al have developed a bamboo rayon fabric grafted with acrylamide utilized to immobilize copper NPs. The product showed antibacterial activity against Gram-positive and Gram-negative bacteria and was found to be durable until 50 washes [94]. Perelshtein et al has recently sonochemically coated a cotton fabric with CuO NPs while maintaining antibacterial properties even after 65 cycles of washings according to hospital protocols of hygienic washing (75 °C) [95]. However, in comparison with AgNPs, higher concentrations of CuO are required to achieve a comparable bactericidal effect [96]. Moreover, CuO NPs synthesis is often more challenging in comparison to noble metals such as silver and gold. Copper sulphate in aqueous solution tends to form Cu2O due to the relatively low CuO/Cu2+ redox potential and spontaneous oxidation of the NPs in ambient conditions. This last drawback can be avoided by protecting copper NPs against oxidation during preparation and storage using non-aqueous solvents, surfactants or ligands to prevent NP agglomeration during the process of synthesis [97].
Other metals and metal oxides
Very few examples are found in the existing literature about the use of other nanomaterials in textiles for antibacterial purposes. Gouda et al has used in situ synthesized zirconium oxide NPs deposited into cotton gauze fabrics. ZrO2 NPs gave a 98% and 95% reduction rate in colony count against Gram-positive and Gram-negative bacteria, respectively. However, antifungal activity was lower than that of fabrics treated with nanosilver. No skin irritation was observed, and all prepared samples were durable enough to wash even after 30 laundering washing cycles [98]. Tang et al developed a simple in situ synthesis route for gold NPs to be applied to multi-functionalized silk fabrics. The AuNPs were prepared in a heated solution containing white silk fabric samples. Silk fabrics treated with AuNPs showed strong antibacterial activity, excellent UV protection properties and enhanced thermal conductivity. However, silk fabrics were colored red and brown by the AuNPs because of their localized surface plasmon resonance property [99]. Harifi et al prepared multi-functional polyester fabric with magnetic, antibacterial and sono-Fenton catalytic activities by in situ synthesis of magnetite and hematite NPs using ferric chloride, ferrous sulphate and sodium hydroxide. The results suggest the potential of the proposed method in producing fabrics with durable magnetic properties that are suitable for various applications such as electromagnetic shielding, antibacterial fabrics and sono-Fenton catalyst for dye discoloration [100]. In their review, Dastjerdi and Montazer discussed other nano-structured, antimicrobial agents with a potential for textile modification, including carbon nanotubes, nanoclay and its modified forms, and gallium- and liposome-loaded NPs; however, no textile applications have yet been developed [32].
Nano-additivated fibers and nanofibers
Several methods also include the bulk modification of conventional filament yarns of polyamide or polypropylene with various concentrations of different nanocomposite fillers, such as Ag, chitosan, PVA, ZnO, TiO2 and mixed Ag/TiO2, via melt mixing [86, 101, 102]. Yeo and Jeong, for example, produced bi-component, sheath-core fibers prepared by using a melt–spinning method with polypropylene chips and AgNPs. However, the fibers containing AgNPs in the core part showed no antibacterial activity. Only fibers having AgNPs in the sheath part showed antibacterial activity [103]. On the other hand, Dastjerdi et al produced biostatic polypropylene filament yarns with various blending contents of nanocomposite based on Ag/TiO2 NPs using a twin-screw extruder. However, despite having good biostatic properties, none of the tested blends displayed a bactericide effect [78]. The bulk modification of filament yarns with various concentrations of nanocomposite fillers via melt mixing is an environmentally friendly and easily adjustable modification method. However, it is limited to synthetic fibers, and the particles situated in the central part of the filaments hardly contribute to the fibers' antibacterial properties. Although the production of core–shell bi-component fibers can be helpful in removing this disadvantage, the required systems are not easily adaptable to industrial standards. A similar problem is also noticeable in the case of reduction of metallic salts to NPs in the bulk polymeric matrix [32].
These problems, however, could be solved by the use of electrospun nanofibers due to their high surface-area-to-volume and length-to-diameter ratios (figure 4) [73, 79, 104–112]. Electrospinning is a process carried out at room temperature that allows the production of polymer fibers with diameters in the sub-micron size range, through the application of an external electric field, keeping intact the bulk properties of the polymers. Because of unique properties such as a high surface-to-volume ratio, very good mechanical performance, high porosity and diameters in the nanoscale, electrospun mats made from ultrafine polymer fibers have been drawing great attention for antimicrobial coatings. Moreover, electrospinning is a high quality, environmentally friendly and easily adjustable method for industrial applications. Several researchers have investigated the spinnability of different polymers. For instance, electrospun nanofibers of cellulose acetate, PVA, PAN and polyester urethane were used to disperse several antimicrobial materials, such as spherical gold and AgNPs, Fe2O3, gallium nitride, zirconium carbide and carbon nanotubes [113–115]. Suspension of AgNPs directly combined into electrospinning polymer solutions is the most used method to prepare composite nanofibers. However, nanofibers produced using this method have demonstrated diminished antimicrobial efficiency due to nanoparticle aggregation. A more efficient method was the in situ reduction of silver ions in pre-electrospinning solutions, resulting in a more uniform dispersion of AgNPs [116].
Figure 4. SEM images (X50000 magnification) of antimicrobial nanofibers obtained from PVA and chitosan (left), and PVA and AgNO3 (right). Images were carried in a FEG-SEM, NOVA 200 Nano SEM, FEI Company. Secondary and backscattering electron images were performed with an acceleration voltage of 5 kV and 15 kV, respectively. Samples were covered with a film of Au–Pd (80–20 wt.%) in a high-resolution sputter coater, 208 h Cressington Company, coupled to a MTM-20 Cressington High Resolution Thickness Controller.
Download figure:
Standard image High-resolution imageElectrospun nanofibers based on chitosan and chitosan NPs applied on several textiles such as cotton, viscose and polyester fabrics have also been extensively investigated. Solutions of pure chitosan are not electrospinnable, independently of their polysaccharide concentrations, mainly due to the high surface tension and conductivities of chitosan acetic acid solutions. Electrospun antimicrobial nanofibers may, however, be fabricated from blended systems of chitosan and fiber-forming polymers such as nylon, cellulose acetate, PEO, PET, PAN and PVA [117–119]. Electrospinning allows extensive tunability in material properties and functions through the selection of polymeric nanofibers, ceramic nanofibers, metallic nanofibers or composite nanofibers. Ideally, nanofibers should be made into continuous yarn before weaving into textile fabrics. However, the diameter of the yarn collected using this process was less than 5 μm and it is uncertain whether the yarn was strong enough to be woven into textiles since several studies showed that insufficient nanofibers in the bundle would result in yarn breakage [120]. For these reasons, now the majority of the electrospun nanofibers incorporating antimicrobial properties are utilized for the production of filtration membranes in order to reduce the formation of biofilm, which is a common source of membrane fouling.
Chitosan
Due to the environmental and toxicological concerns about the use of heavy metals for the production of NPs, researchers have been recently exploring the use of natural polymers, especially chitosan, in the antimicrobial finishing of textiles. Chitosan [(C6H11O4N)n], the N-deacetylated derivative of chitin [(C8H13O5N)n] due to the presence of amino groups, is a cationic polyelectrolyte, one of the few occurring in nature. This gives chitosan singular chemical and biological characteristics, such as biocompatibility, antibacterial properties, heavy metal ion chelation ability, gel-forming properties and hydrophilicity. The use of chitosan NPs in protein and drug delivery systems is being actively researched and reported in the literature [121]. However, the research on chitosan NPs for textile applications is limited because most of the literature is based on the use of bulk chitosan as a coating or finishing agent. Antimicrobial fabrics with nanocoated chitosan have proved to be a durable, cost-effective and eco-friendly process. Some research has shown, however, that chitosan NPs have a less inhibiting effect on S. aureus compared to bulk chitosan since NPs have less positive charge available to bind to the negative bacterial cell wall. Conversely, other researchers reported that chitosan NPs exhibit higher antibacterial activity due to the NP's larger surface area and higher affinity with bacteria cells, which yield a quantum-size effect [53, 122–125]. These contradictory results suggest that the antimicrobial mode of action of chitosan is not a simple mechanism, but is an intricate event-driven process that needs further investigation [126].
Conclusions
Most of the literature about antimicrobial textile nanocomposites is focused on silver. However, other metals and metal oxides such as zinc, titanium, copper, zirconium, iron and gold show improved biocidal properties at nanoscale. ZnO and CuO nanocomposites display similar performance compared to silver while TiO2 efficacy is limited by light availability due to its photocatalytic mechanism of action. Despite the heterogeneous range of methods, textile substrates, nanoparticle sizes and concentrations that can be found in the literature, some general assumptions can be made about metal and metal oxide NPs based on the collected data. Silver and copper NPs of between 1 and 15 nm showed the best biocide activity at relatively low concentrations on the fabrics (5–50 ppm or 1–2 wt.%). AgNPs of up to 50 nm, still require relatively low concentrations of around 100 ppm or 5 wt.% to have complete Gram-positive and Gram-negative inhibition effects. Titanium oxide NPs applied to textiles are generally in the size range of 1–20 nm. With some exceptions, TiO2 NPs showed low antimicrobial activity (an average of 70%) even at high concentrations of 10 wt.% on the fabrics. This occurs mainly because TiO2 NPs are fully effective just under UV rays, which limits their practical use in the textile industry. On the other hand, ZnO NPs need a higher average size of 30–40 nm, but with a lower concentration (around 1 wt.%) than TiO2 to be effective. This is possibly due to the synergetic dual effect of the photocatalytic generation of hydrogen peroxide and the direct disorganization of the bacterial membrane. However, despite the promising results, the available information of ZnO NPs on textiles is still limited. All types of metal and metal oxide NPs with diameters greater than 100 nm need concentrations comparable to the metal ions or bulk materials to achieve the same antimicrobial performance.
The bacterial species Staphylococcus aureus (Gram positive), Escherichia coli (Gram negative) and Klebsiella pneumoniae (Gram negative) are the most-tested strains. Some authors have also tested different bacteria and fungi such as Candida albicans. However, the eukaryotic cytotoxicity and allergic reactions in humans are not considered in NP-containing textiles. Moreover, few authors have tested the antimicrobial efficiency after a reasonable number (at least 20) of washing cycles, limiting the precise estimation of the amount and form of NPs released from the fabrics into the environment. The risk assessment of the nanomaterials used in commercial textile products requires a better understanding of nanomaterial mobility, bioavailability and toxicity in the environment. Due to this increasing dichotomy between environmental and health concerns and the potential benefits of using NPs as an antimicrobial finish for textiles, the use of natural polymers, especially chitosan, and electrospun nanofibers have been recently explored. The research about chitosan NPs deposited on fabrics is still at an early stage; however, from the little information available, it is possible to estimate that the average sizes range from 20–200 nm and that the effective concentration is usually lower than 1 wt.%. The latest research in this field seems to indicate an emerging new paradigm in the production and distribution of NPs for textile applications utilizing non-toxic renewable biopolymers such as chitosan, alginate and starch.
Acknowledgement
Andrea Zille (C2011-UMINHO-2C2T-01) acknowledges funding from Programa Compromisso para a Ciência 2008, Portugal.
Author contributions
Andrea Zille performed the AgNP synthesis, AgNP deposition on polyamide fabrics, SEM and TEM analysis of the AgNPs and nanofibers, analyzed the data, interpreted results, elaborated table 1 and wrote the review. Noémia Carneiro performed the ZnO NPs synthesis, ZnO NPs deposition on cotton fabrics, SEM analysis of the deposited ZnO NPs, analyzed data, interpreted results, help in the table elaboration and reviewed the manuscript. António Pedro Souto performed the DBD plasma pre-treatment of the fabrics, analyzed data, interpreted results, helped with the table elaboration and reviewed the manuscript. Carla J Silva has prepared the electrospun nanofibers. Luís Almeida, Teresa Amorim and Maria Fátima Esteves analyzed data, interpreted results, helped with the table elaboration and reviewed The manuscript.