Abstract
Perovskite-structured Li0.375Sr0.4375Hf0.25Ta0.75O3 is regarded as a promising solid electrolyte for all solid state Li ion batteries. In this work, the effect of trivalent ion (La3+, Sm3+ and Al3+) doping on crystalline structure and conductivity for Li0.375Sr0.4375Hf0.25Ta0.75O3 solid electrolyte is investigated. X-ray diffraction measurement confirms that Li0.375Sr0.4375Hf0.25Ta0.75O3 presents cubic perovskite structure with space group of Pm-3m. Al-doped Li0.375Sr0.4375Hf0.25Ta0.75O3 has pure cubic perovskite structure. But, the doping of La3+ and Sm3+ leads to formation of impurity phases such as La2Hf2O7 and Sm2Hf2O7. AC-impedance analysis indicates that trivalent ion doping leads to decrease of conductivity at room temperature. Among all compounds, Li0.375Sr0.4075La0.02Hf0.25Ta0.75O3 has the lowest activation energy of 0.318 eV.
Export citation and abstract BibTeX RIS
1. Introduction
Perovskite-type Li-ion electrolytes have been considered as potential solid state electrolytes for next general Li ion battery [1]. Li3xLa2/3−xTiO3 (LLTO) is a typical Perovskite-type Li-ion solid electrolyte, its bulk conductivity can reach 10−3 S cm−1 [2]. However, total conductivity of LLTO is still very low due to huge grain-boundary resistance [3]. Searching for new Li-ion solid electrolytes with high total conductivity has attracted much attention [4]. Designing solid electrolytes based on perovskite structure is an effective approach [5]. To take ABO3-type perovskite compounds for an example, the addition of Li+ into A-sites introduces mobile Li ions. At the same time, the addition of high-valent ions into B-sites introduces cationic vacancies [6, 7]. In previous reports, perovskite-structured Li–Sr–Zr–Ta–O (Li–Sr–Zr–Nb–O) and Li–Sr–Hf–Ta–O (Li–Sr–Hf–Nb–O) were designed and prepared base on perovskite-type SrZrO3 and SrHfO3 compounds, respectively. The total conductivity of Li0.375Sr0.4375□0.1875Zr0.25Ta0.75O3 and Li0.375Sr0.4375□0.1875Hf0.25Ta0.75O3 is as high as 2.0 × 10−4 S cm−1 and 3.3 × 10−4 S cm−1, respectively [6–10]. But their conductivities are still low compare to practical requirement (10−3 S cm−1). Further improving conductivity is important for these solid electrolytes.
An effective approach for improving property of materials, increasing conductivity of presenting solid electrolytes and designing new solid electrolytes is to optimize Li+/cationic vacancies ratio through ion doping [11]. In solid electrolyte, conducting species is Li ion only and the electronic conductivity is negligible. So conductivity of these materials can be given as  . Here,
. Here,  is the conductivity, n is density of mobile ion,
is the conductivity, n is density of mobile ion,  is the charge of Li ion and
is the charge of Li ion and  is the mobility of Li ions.
is the mobility of Li ions.  , where k is the Boltzmann constant, T is temperature and D is the diffusion coefficient. D is related to the number of cationic vacancy [3]. Combine the two equations, Nernest–Einstein equation can be obtained:
, where k is the Boltzmann constant, T is temperature and D is the diffusion coefficient. D is related to the number of cationic vacancy [3]. Combine the two equations, Nernest–Einstein equation can be obtained:  . As can be seen, the conductivity is proportional to both of the density of mobile ion and the diffusion coefficient [3, 12–14]. Li ions and cationic vacancies can be introduced by low-valent and high-valent doping, respectively [15–17]. So aliovalently doping is an effective way to improve ionic conductivity of crystalline solid electrolytes. All in all, we can design new solid state Li ion conductors or improve conductivity of presenting solid electrolytes base on ion doping.
. As can be seen, the conductivity is proportional to both of the density of mobile ion and the diffusion coefficient [3, 12–14]. Li ions and cationic vacancies can be introduced by low-valent and high-valent doping, respectively [15–17]. So aliovalently doping is an effective way to improve ionic conductivity of crystalline solid electrolytes. All in all, we can design new solid state Li ion conductors or improve conductivity of presenting solid electrolytes base on ion doping.
Li0.375Sr0.4375□0.1875Hf0.25Ta0.75O3 (LSTH) is a promising solid electrolyte for all solid state Li-ion batteries. Its conductivity is as high as 3.3 × 10−4 S cm−1 at room temperature. Further improving its conductivity is important. Therefore, Xu et al [10] investigated the influence of sintering additives (Li3BO3, Li4SiO4, LiF) on the total conductivity of LSTH. Sintering additives can improve density of LSTH and then facilitate Li ion transport across grain-boundary [10, 18, 19]. However, effect of doping on the structure and electrical properties of LSTH was not investigated.
In ABO3–type perovskite-structured Li0.375Sr0.4375Hf0.25Ta0.75O3 solid electrolyte, (Li0.375Sr0.4375) occupy A-sites while (Hf0.25Ta0.75) occupy B-sites. The doping of trivalent ions for bivalent Sr at A-site can introduce cationic vacancies. The doping of trivalent ions for tetravalent ions at B-site can introduce Li ions.
In this work, we designed and synthesized Al-doped LSTH, La-doped LSTH and Sm-doped LSTH solid electrolytes. Their formulas are [Li0.375Sr0.4375]1+1.75x[Hf0.25Ta0.75]1−xAlxO3, Li0.375[Sr0.4375−1.5xLax]Hf0.25Ta0.75O3, and Li0.375[Sr0.4375−1.5xSmx]Hf0.25Ta0.75O3, respectively. Values of x for each sample were listed in table 1. We studied structure and electrical property of aliovalently doped Li0.375Sr0.4375Hf0.25Ta0.75O3 solid electrolyte, in which Li ions or new cationic vacancies are introduced.
Table 1. Composition of compounds.
| Compounds | Compositions | Value of x |
|---|---|---|
| LSTH | Li0.375Sr0.4375Hf0.25Ta0.75O3 | — |
| LSTH-Al-1 | [Li0.375Sr0.4375]1+1.75x[Hf0.25Ta0.75]1−xAlxO3 | 0.05 |
| LSTH-Al-2 | [Li0.375Sr0.4375]1+1.75x[Hf0.25Ta0.75]1−xAlxO3 | 0.1 |
| LSTH-La-1 | Li0.375[Sr0.4375−1.5xLax]Hf0.25Ta0.75O3 | 0.02 |
| LSTH-La-2 | Li0.375[Sr0.4375−1.5xLax]Hf0.25Ta0.75O3 | 0.05 |
| LSTH-Sm-1 | Li0.375[Sr0.4375−1.5xSmx]Hf0.25Ta0.75O3 | 0.02 |
| LSTH-Sm-2 | Li0.375[Sr0.4375−1.5xSmx]Hf0.25Ta0.75O3 | 0.05 |
2. Experimental
All samples were prepared via solid state reaction. Compositions of each sample were listed in table 1. All agents were purchased from GUOYAO Co Ltd. Stiochiometric amounts of SrCO3, Ta2O5, Li2CO3 (20 weight% excess), HfO2, Al2O3, La2O3 and Sm2O3 were weighed and then mixed by ball milling with alcohol as solvent. Mixtures were calcined at 1100 °C for 12 h to obtain powder. The powder were pressed into pellets (Φ = 15 mm) by isostatic pressing at 200 Mpa for 9 min. All pellets were covered by corresponding mother powder and were sintered at 1300 °C for 15 h in air.
Before testing, all pellets were polished by sandpaper of 2000 grids. Phase composition and crystalline structure were detected at room temperature by x-ray diffraction (XRD) using X pert PRO (Panalytiacl) with Cu Kα radiation. Lattice parameters of solid electrolyte were evaluated by XRD. The apparent densities of ceramic pellets were estimated by using Archimedes' method. The cross-section morphologies of ceramic pellets were characterized by scanning electron microscope (SEM) technology on FEI-250. The electronic conductivity of compounds was estimated by potentiostatic polarization experiment at room temperature, using Princeton-V3 electrochemical workstation.
Conductivities of all compounds were detected by using AC-impedance technology with an applied voltage of 50 mV on Solartron 1260 Impedance/Gain-Phase Analyzer. Gold paste was coated on both faces of ceramic pellets and then calcined at 750 °C for 2 h as electrode. The frequency range was from 1 Hz to 10 MHz. Temperature dependence of total conductivity was measured in the temperature range from 15 °C to 120 °C.
3. Results and discussion
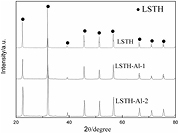
Figure 1 shows XRD patterns of undoped LSTH and Al-doped compounds. As can be seen, perovskite-structured LSTH was synthesized successfully by conventional solid reaction method. This crystalline structure agrees with related reports [9, 10]. As can be seen in figure 1, Al-doped LSTH compounds have the same perovskite structure with pure LSTH. In the ABO3-type perovskite structure of LSTH, Li and Sr ions occupied A-sites while Hf and Ta ions occupied B-sites. The ionic radius of Al3+ is 0.535 Å, which is smaller than Hf4+ (0.71 Å) and Ta5+ (0.64 Å). As a result, diffraction peaks of Al-doped compounds shift toward higher angle. This is correspond with figure 1, indicates that Al3+ was doped into LSTH successfully. The substitution of Hf4+ and Ta5+ by Al3+ leads to a decrease of lattice parameter (listed in table 2). The decreases of lattice parameter lead to decrease of bottleneck size, which is not good for conductivity. As can be seen in figure 1, impurity phases were not detected after doping Al3+, indicates that there is no side reaction during high temperature sintering.
Table 2. Lattice parameter, density, and conductivity and activation energy of compounds.
| Compounds | Lattice parameter/Å | Density/g cm−3 | σ30 °C/mS cm−1 | Activation energy/eV |
|---|---|---|---|---|
| LSTH | 3.992(1) | 6.47 | 0.337 | 0.338 |
| LSTH-Al-1 | 3.983 | 6.11 | 0.063 | 0.370 |
| LSTH-Al-2 | 3.977 | 6.02 | 0.037 | 0.377 |
| LSTH-La-1 | 4.001(9) | 6.44 | 0.334 | 0.318 |
| LSTH-La-2 | 4.001(3) | 6.40 | 0.225 | 0.323 |
| LSTH-Sm-1 | 4.000(7) | 6.37 | 0.237 | 0.324 |
| LSTH-Sm-2 | — | 6.33 | 0.0145 | 0.343 |
Figure 1. XRD patterns of undoped LSTH and Al-doped compounds.
Download figure:
Standard image High-resolution imageFigures 2(a) and (b) shows XRD patterns of La- and Sm-doped LSTH. La3+ (1.03 Å) and Sm3+ (0.958 Å) have smaller ionic radii than Sr2+ (1.18 Å). But the main peaks of La, Sm-doped LSTH shifted toward lower angles. It means that lattice size increased after doping La and Sm. It's likely that a part of Li+ with small radii (0.76 Å) were substituted by La3+ and Sm3+. LSTH-La-1 presents pure perovskite structure as well as LSTH. When further increasing doping amount of La, impurity phases such as Ta2O5 and pyrochlore-type La2Hf2O7 were detected. For Sm-doped LSTH, it is difficult to obtain pure perovskite structure. Impurities such as pyrochlore-type Sm2Hf2O7 were detected in all samples. Intensity of diffraction peaks of impurity phase increases with increasing doping amount. The presence of impurity phases may be attributed to the volatilization of Li elements during high temperature sintering. Furthermore, the small radii of Sm3+ (0.958 Å) compare with Sr2+ (1.18 Å) may be another important reason. For Sm-doped compounds, it is difficult to obtain pure perovskite phase even the doping contents was very small (x = 0.02). It seems that the Sm element itself cannot form a doping. These impurities could not conduct Li ions, which is not beneficial to ionic conduction. This point can be confirmed by corresponding AC-impedance experiment.
Figure 2. XRD patterns of La-doped LSTH (a) and Sm-doped LSTH (b).
Download figure:
Standard image High-resolution imageFigure 3 shows the typical SEM images of cross-section of ceramic pellets. The well-crystallized grains were observed and all grains contacted well with neighboring grains. As can be seen in figure 3(a), LSTH has a dense microstructure. But there are still amount of pores in ceramic pellets. Presence of pores can decrease density of pellets, which is not benefit to ionic conduction. Among all pellets, LSTH-La-1 has the largest grain size. Impurities were observed in LSTH-La-2, LSTH-Sm-1 and LSTH-Sm-2, which is corresponding to their XRD patterns.
Figure 3. SEM images of cross-section of LSTH (a) and (b), LSTH-Al-1 (c), LSTH-Al-2 (d), LSTH-La-1 (e), LSTH-La-2 (f), LSTH-Sm-1 (g) and LSTH-Sm-2 (h).
Download figure:
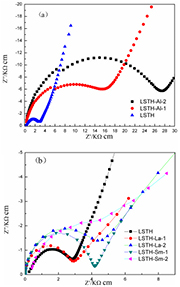
Standard image High-resolution imageConductivities of all ceramic pellets were determined by AC-impedance technology. Au electrode was used as ionic blocking electrode for AC-impedance measurement. Figure 4(a) shows AC-impedance spectra of LSTH and Al-doped compounds. As can be seen, both of semicircle in high frequency region and straight line in low frequency region were obtained. But, only one semicircle was observed in high frequency region. This semicircle represents total resistance of solid electrolytes. AC-impedance data were fitted by using equivalent circuit (QR) (QR) (R(CW)). Total conductivity of solid state electrolytes was calculated by using following equation  . Here, d is thickness of ceramic pellets, A is surface area of electrode and R is the total resistance, respectively. As can be seen in figure 4(a), total resistance of solid electrolyte increases with increasing Al contents. Conductivities of all compounds were calculated and listed in table 1. As a result, conductivity of solid electrolyte displays negative correlation with Al contents. In our opinion, the reason for presenting lower conductivity in Al-doped LSTH is that optimized Li+/cation vacancies ratio is not obtained [3, 7, 13]. By Al3+ doping, cationic vacancy concentration decreases due to charge compensation [20]. The cationic vacancy concentration is important parameters for Li ions conduction in LSTH. In LSTH, the conductive ion is Li+ only. Since the doping of Al introduces Li ions into LSTH, the sharp decrease of ionic conductivity may be attributed to the decrease of cationic vacancies. Furthermore, the bottleneck size of LSTH perovskite decreases with the addition of Al3+, which is not beneficial to ion conduction.
. Here, d is thickness of ceramic pellets, A is surface area of electrode and R is the total resistance, respectively. As can be seen in figure 4(a), total resistance of solid electrolyte increases with increasing Al contents. Conductivities of all compounds were calculated and listed in table 1. As a result, conductivity of solid electrolyte displays negative correlation with Al contents. In our opinion, the reason for presenting lower conductivity in Al-doped LSTH is that optimized Li+/cation vacancies ratio is not obtained [3, 7, 13]. By Al3+ doping, cationic vacancy concentration decreases due to charge compensation [20]. The cationic vacancy concentration is important parameters for Li ions conduction in LSTH. In LSTH, the conductive ion is Li+ only. Since the doping of Al introduces Li ions into LSTH, the sharp decrease of ionic conductivity may be attributed to the decrease of cationic vacancies. Furthermore, the bottleneck size of LSTH perovskite decreases with the addition of Al3+, which is not beneficial to ion conduction.
Figure 4. Nyquist plots of Al-doped LSTH (a) and La-, Sm-doped samples (b).
Download figure:
Standard image High-resolution imageFigure 4(b) shows Nyquist plots of La- and Sm-doped compounds. As can be seen, the conductivity decreases slightly after doping La3+ and Sm3+. By doping Sm3+ and La3+ for Sr2+ in Li0.375Sr0.4375Hf0.25Ta0.75O3 ceramics, cationic vacancy concentration increases due to charge compensation. In theory, the conductivity of solid state Li ion conductor would be a positive correlation with cationic vacancy concentration [19]. But as can be seen, conductivities of LSTH-La-2, LSTH-Sm-1 and LSTH-Sm-2 are lower than LSTH. Especially, conductivity decreased obviously after doping Sm3+. This may be attributed to the presence of impurity phases. As can be seen in XRD patterns, the main impurities are Ta2O5, pyrochlore-type La2Hf2O7 and Sm2Hf2O7. These impurity phases do not conduct Li ions, which can block the migration of Li+, and then decrease ionic conductivity. LSTH-La-1 has similar ionic conductivity with LSTH at room temperature. Actually, the conductivity of LSTH-La-1 is higher than LSTH below 30 °C. This is attributed to the avoidance of impurities and increasement of cationic vacancies.
Whether it is over conductivity 10−3 S cm−1 at room temperature is important for evaluate solid electrolytes. As can be seen, the total conductivity of LSTH-based solid electrolytes is still below 10−3 S cm−1 at room temperature in this work. Actually, perovskite-structured Li0.375Sr0.4375Hf0.25Ta0.75O3 has the potential to achieve 10−3 S cm−1, and many efforts such as adding sintering additives, optimizing compositions and doping have been made to reach this target [10, 15, 22, 23]. But all these efforts seem to be failure. In LSTH, the contribution of grain boundary resistance is lower than grain resistance. In other words, bulk conductivity is the limiting factor. So, doping may be an effective way to improve conductivity for LSTH. Based on previous reports and this work, ion doping in B-site with large radii (larger than Hf4+, Zr4+ and Ta5+) to expand bottleneck size, ion synergistic doping in both A- and B-sites to created cationic vacancy/interstitial Li ions and combining the advantages of sintering additives and doping to improve both grain and grain boundary conductivity are promising approaches to further improve total conductivity of LSTH.
Temperature dependence of total conductivity for all compounds was measured in the temperature range from 15 to 120 °C. According to Arrhenius equation  , activation energies of conductivity for solid electrolytes can be derived [21, 22]. Here,
, activation energies of conductivity for solid electrolytes can be derived [21, 22]. Here,  is activation energy, T is absolute temperature,
is activation energy, T is absolute temperature,  is constant and
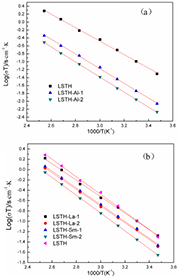
is constant and  is Boltzmann constant (=1.381 × 10−23 J K−1). Figures 5(a) and (b) shows variation of total conductivity of Al-, La- and Sm-doped LSTH as a function of reciprocal of temperature. Values of activation energy for all samples were listed in table 1. LSTH has the activation energy of 0.338 eV. After doping Al into LSTH, an obvious increase in activation energy was observed. Ea of LSTH increases with increasing Al contents. Activation energies of LSTH-Al-1 and LSTH-Al-2 are 0.370 and 0.377 eV, respectively. This is attributed to the decrease of cationic vacancy concentration and bottleneck size. Activation energies of LSTH-La-1, LSTH-La-2 and LSTH-Sm-1 are lower than LSTH. Among all compounds, LSTH-La-1 has the lowest activation energy of 0.318 eV. This is attributed to the increasement of cationic vacancy concentration.
is Boltzmann constant (=1.381 × 10−23 J K−1). Figures 5(a) and (b) shows variation of total conductivity of Al-, La- and Sm-doped LSTH as a function of reciprocal of temperature. Values of activation energy for all samples were listed in table 1. LSTH has the activation energy of 0.338 eV. After doping Al into LSTH, an obvious increase in activation energy was observed. Ea of LSTH increases with increasing Al contents. Activation energies of LSTH-Al-1 and LSTH-Al-2 are 0.370 and 0.377 eV, respectively. This is attributed to the decrease of cationic vacancy concentration and bottleneck size. Activation energies of LSTH-La-1, LSTH-La-2 and LSTH-Sm-1 are lower than LSTH. Among all compounds, LSTH-La-1 has the lowest activation energy of 0.318 eV. This is attributed to the increasement of cationic vacancy concentration.
Figure 5. Arrhenius plots for total conductivity of Al, La and Sm-doped LSTH.
Download figure:
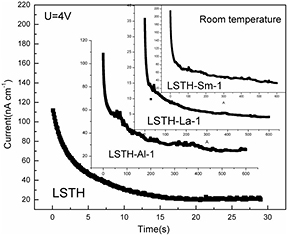
Standard image High-resolution imageElectronic conductivity ( ) is an important property for solid electrolyte as well as ionic conductivity. Electronic conductivity can be estimated by using potentiostatic polarization measurement. Figure 6 shows potentiostatic polarization curves of LSTH, LSTH-Al-1, LSTH-La-1 and LSTH-Sm-1. The electronic conductivity can be calculated by following equation
) is an important property for solid electrolyte as well as ionic conductivity. Electronic conductivity can be estimated by using potentiostatic polarization measurement. Figure 6 shows potentiostatic polarization curves of LSTH, LSTH-Al-1, LSTH-La-1 and LSTH-Sm-1. The electronic conductivity can be calculated by following equation  [23], where L is the thickness of ceramic pellets, I is static current, U is applied voltage and A is the area of Au electrode. Electronic conductivity can be obtained from the static current shown in figure 6. As a result, the electronic conductivity of LSTH is about 5.04 × 10−9 S cm−1, which is 105 times lower than its ionic conductivity. With the addition of Al and Sm, electronic conductivities of solid electrolyte increase slightly. The electronic conductivity and the ratio of its contribution for each sample were calculated and were listed in table 3.
[23], where L is the thickness of ceramic pellets, I is static current, U is applied voltage and A is the area of Au electrode. Electronic conductivity can be obtained from the static current shown in figure 6. As a result, the electronic conductivity of LSTH is about 5.04 × 10−9 S cm−1, which is 105 times lower than its ionic conductivity. With the addition of Al and Sm, electronic conductivities of solid electrolyte increase slightly. The electronic conductivity and the ratio of its contribution for each sample were calculated and were listed in table 3.
Table 3. Total conductivity and electronic conductivity of compounds.
| Samples | Total conductivity/S cm−1 | Electronic conductivity/S cm−1 | 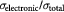 |
|---|---|---|---|
| LSTH | 3.37 × 10−4 | 5.04 × 10−9 | 1.49 × 10−5 |
| LSTH-Al-1 | 0.63 × 10−4 | 5.21 × 10−9 | 8.26 × 10−5 |
| LSTH-La-1 | 3.34 × 10−4 | 1.01 × 10−9 | 0.30 × 10−5 |
| LSTH-Sm-1 | 2.37 × 10−4 | 12.1 × 10−9 | 5.10 × 10−5 |
Figure 6. Potentiostatic polarization curves of Al, La and Sm-doped LSTH.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, Al, La and Sm-doped Li0.375Sr0.4375Hf0.25Ta0.75O3 were prepared via solid state reaction followed by a high temperature sintering process at 1300 °C. Al-doped LSTH presents pure cubic perovskite structure as well as Li0.375Sr0.4375Hf0.25Ta0.75O3. Doping of La and Sm introduced impurity phases. LSTH has a high ionic conductivity of 3.37 × 10−4 S cm−1 at room temperature with activation energy of 3.338 eV. Electronic conductivity of LSTH is 5 orders of magnitude lower than its ionic conductivity, indicates that LSTH is a Li ions conductor in nature. AC-impedance analysis investigates that trivalent ion doping lead to decreases of conductivity at room temperature. But La-doped sample Li0.375Sr0.4075La0.02Hf0.25Ta0.75O3 has higher conductivity than pure LSTH at 15 °C. The decrease of conductivity after doping Al is attributed to the decrease of cationic vacancy concentration and bottleneck size. The decrease of conductivity after doping Sm is attributed to the presence of insulating impurities. Among all compounds, La doped compound LSTH-La-1 has the lowest activation energy of 0.318 eV, which is attributed to the increasement of cationic vacancy concentration after doping La3+.
Acknowledgments
This work was supported by the National Science Foundation of China (NSFC-No. 51474057, No. 51274057) and the Fundamental Research Funds for the Central Universities (N162502003).