Abstract
The effect of pressure variation on stability, structural parameters, elastic constants, mechanical, electronic and thermodynamic properties of cubic SrKF3 fluoroperovskite have been investigated by using the full-potential linearized augmented plane wave (FP-LAPW) method combined with Quasi-harmonic Debye model in which the phonon effects are considered. The calculated lattice parameters show a prominent decrease in lattice constant and bonds length with the increase in pressure. The application of pressure from 0 to 25 GPa reveals a predominant characteristic associated with widening of bandgap with GGA and GGA plus Tran–Blaha modified Becke–Johnson (TB-mBJ) potential. The influence of pressure on elastic constants and their related mechanical parameters have been discussed in detail. Apart of linear dependence of elastic coefficients, transition from brittle to ductile behavior is also observed at elevated pressure ranges. We have successfully computed variation of lattice constant, volume expansion, bulk modulus, Debye temperature and specific heat capacities at pressure and temperature in the range of 0–25 GPa and 0–600 K.
Export citation and abstract BibTeX RIS
1. Introduction
Now-a-days the crystals of fluoroperovskites are becoming increasingly useful because of their relatively simple crystalline structure and due to their wide bandgaps. The physical properties of these materials have been the subject of many new applications, involving new fields of material science. The important domains of application include Piezoelectric systems, electric ceramics, optical parametric oscillators, solar energy convertors, low birefringent lenses, optical wave guides, and high temperature sensors. In particular, the SrKF3 compound attains large industrial and technological interest in optical lithography steppers because it has wide and direct bandgap and thus preferable for lens fabrication technology and transparent optical coatings [1–4].
An experimental study on BaLiF3 and SrLiF3 compounds are carried out by Duvel and its fellows [5] shows that this series of materials exhibits some special symmetries of phase transition in the presence of metastable quaternary fluorides. Chadwick et al [6] reports the results of fast-ionic conduction in KCaF3 at high temperatures. Theoretically, there are few computational studies based on common density functional theory (DFT) approximations such as local density approximation (LDA) and generalized gradient approximation (GGA) within first principles technique [7–13]. However we, for the first time, reported in our recent work [14, 15] the rigidity, mechanical behavior and opto-electronic trend of SrMF3 (M = Li, Na, K, Rb) compounds at fixed (zero) pressure and fixed (0 K) temperature by using ab initio first principles technique. As a result, we found many interesting physical properties in this series of compound. Unfortunately, in the available literature, as per best of our knowledge no one have explore SrKF3 compound at different pressure and different temperature ranges. Though pressure imposes significant variation on important physical properties such as lattice parameters, electronic density of states (DOS), Band structure curves, elastic moduli's, cubic stability conditions, complex dielectric coefficients, refractive index, reflectivity and so on. The lack of available results of concerning physical properties motivated us to extend our previous analysis under different pressure and higher temperature ranges. Here we considered to provide, a complementary and comparative study of our own and some experimental works by examining structural, elastic, mechanical, electronic, optical and thermodynamic properties of cubic SrKF3 fluoro-perovskite compound under fixed as well as varying pressure (0–25 GPa) and varying temperature (0–600 K). According to the best of our literature survey, it is firstly reported work of this nature.
The sections in the paper are organized as follows: section 2 describes calculation methodology. Section 3 presents the results and discussions of structural, elastic, mechanical, electronic, optical and thermodynamic properties under various pressure and temperature ranges and all results are compared where data is available. In the last section, we conclude the results of the present research which helps us to enlighten future application of this investigation.
2. Computational details
In the present work, all calculations have been done using the wien2k package of ab initio simulation based on DFT full-potential linearized augmented plane wave (FP-LAPW) method [16–17]. For the structural optimization the exchange-correlation contribution is described within the local density approximation (LDA) and generalized gradient approximation as proposed by Wu and Cohen (WC-GGA), however electronic analysis has been done via a variety of exchange correlation energy functionals E(xc), such as the LDA, Perdew–Burke–Ernzerhof generalized gradient approximation (PBE-GGA), Wu Cohen generalized gradient approximation (WC-GGA), and PBEsol-GGA, but we highlight the results of the modified Becke–Johnson potential (mBJ). Details of these exchange correlation phenomenon can be found in [18–22]. The sphere radii used in the corresponding calculations are  respectively. The plane wave cut-off can be found to be adequate at −7 Ry. The 12 × 12 × 12 grid mesh of Brillouin zone (BZ) is integrated using tetrahedron method [23, 24]. Thus, this mesh ensures the convergence of total energy and charge to be less than 0.001 mRy and 0.1 m respectively. Furthermore, to obtain thermodynamic properties, we induce Quasi-harmonic Debye model, in which we implement non-equilibrium Gibbs function [25]. In this way the Gibbs free energy G (V, P, T) is obtained to minimize G for deriving the thermal equation of state (EOS) V (P, T). Further, macroscopic parameters as a function of pressure and temperature are derived from standard thermodynamic relations [26, 27]. The detailed description of the Quasi-harmonic Debye model was found in [28–34].
respectively. The plane wave cut-off can be found to be adequate at −7 Ry. The 12 × 12 × 12 grid mesh of Brillouin zone (BZ) is integrated using tetrahedron method [23, 24]. Thus, this mesh ensures the convergence of total energy and charge to be less than 0.001 mRy and 0.1 m respectively. Furthermore, to obtain thermodynamic properties, we induce Quasi-harmonic Debye model, in which we implement non-equilibrium Gibbs function [25]. In this way the Gibbs free energy G (V, P, T) is obtained to minimize G for deriving the thermal equation of state (EOS) V (P, T). Further, macroscopic parameters as a function of pressure and temperature are derived from standard thermodynamic relations [26, 27]. The detailed description of the Quasi-harmonic Debye model was found in [28–34].
3. Results and discussion
3.1. Structural and electronic properties
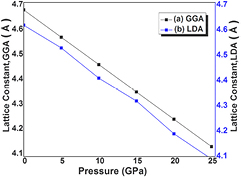
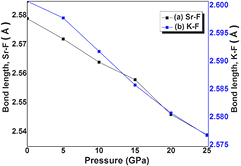
The SrKF3 compound has an ideal cubic structure with space group Pm-3 m (no. 221). In our recent work [14], we have calculated ground state structural parameters by structural relaxation using Birch Murghan's equation of state. The details of optimized lattice parameters such as lattice constants, tolerance factor, bond lengths, bulk modulus, and its pressure derivative at fixed pressure and zero temperature can be found in our earlier published work [15]. However, for the convenience of new reader we have mentioned modified and precise form of that work in table 1. For the lattice constant (ao) about 4% deviation is observed between experimental and present calculation that is obvious, because the experimental work was done at ambient conditions while the present work is done at zero kelvin and Castro [29], uses large volume of unit cell as compared to the present first principle investigation. Our current task is to analyze effect of pressure on structural and electronic parameters of SrKF3 compound with 0–25 GPa range with a step size of 5GPa. The hydrostatic pressure of 0.00 GPa is considered as a base for ground state stable structure and increase in pressure further decreases the volume of the crystal structure as shown in table 1. The calculated lattice constants by LDA and GGA approximation schemes and bond lengths are plotted in figures 1 and 2 respectively. It can be analyzed from the figures that both lattice constants and bond lengths are going to decrease as pressure increases in accordance with our previous work/Other work [7, 9, 14, 15] (at ambient pressure). The key issue to discuss the electronic structure of SrKF3 is the formation and widening of bandgap with the increase in pressure. As it can be observed from table 2 that compression on the system (0 GPa–25 GPa) increases the bandgap from 3.307 eV to 3.845 eV from GGA scheme and 6.799 eV to 7.302 eV from mBj potential as shown in figure 3. This change in the value of bandgap is in continuation with our previous study [15], that GGA and LDA schemes undervalues energy bandgap of wide bandgap semiconductors and insulators. The cause of this underestimation is discussed in detail in [19]. The calculated lattice constants, bond lengths and volume of unit cell compress to a reasonable extent that the constituent polyhedral of SrKF3 do not become distorted by the change in pressure from 0–25 GPa. In general, the opening of wide bandgap in fluoroperovskites are due to electro-negativity of fluorine ion. Similar to oxide perovskites, fluorine based perovskites also form the weak covalent bond [35–38]. In this regard, the pressure induced behavior of SrKF3 is in good agreement with previously reported work by Lee et al [39], that decrease in bond length increases the bonding energy as strength of covalent bond decreases, consequently wider bandgap. In this work, we have examined that as compression increases there is decrease in bond lengths both for Sr–F and K–F as shown in figure 2 respectively. While according to table 2, the calculated bond lengths are in reasonable agreement with the previously published work at 0 GPa. The DOS and energy band structures at different pressure ranges from 0–25 GPa are shown in figures 3–5. We have observed that throughout the calculations the position of maxima of valence band F-2p states remains almost same. The total and partial density of states (TDOS & PDOS) occupies energy interval from (EF − 30 eV) up to (EF + 15 eV) at ambient pressure as shown in figure 5. It can be observed that above fermi level slightly wide peak is dominated by Sr-3d state at about (EF + 8 eV). Next energy interval (EF − 3 eV) is governed by F-2p state. The upper valence band situated in a range from (EF − 12 eV) to (EF − 20 eV) is due to hybridized state of Sr-4p, K-2p and F-2s states respectively. So, the cubic SrKF3 is by virtue, a very wide bandgap modern semiconductor and with the increase in the pressure the strength of hybridization increases with reduced bond lengths which give rise to antibonding phenomenon among bonds. This antibonding creates high energy, which pushed up energy level away from Ef, consequently widening of bandgap occurs which is previously reported in Pseudo potential theory [40, 41] as well.
Table 1. Comparison of the Present calculation with the previous experimental and theoretical values for the lattice constants (ao), ground state energies (Eo), bulk modulus (Bo) and its pressure derivative (B') at ambient pressure of SrKF3 compound.
| Compound: SrKF3 | Present work | Previous work |
|---|---|---|
| ao (Å) | 4.678 | 4.680 |
| Bo (GPa) | 38.698 | 37.692 |
| B' (GPa) | 2.961 | 2.991 |
aMubarak and Mousa [7]. bMubarak [14]. cErum and Iqbal [15] (other theoretical work). dCastro [29] (experimental work).
Table 2. Comparison of previous and calculated values of pressure (P in GPa), energies (E in Ry), volume of unit cell (V in (a.u.)3), energy gap (eV), and bond length (dSr–F, dK–F).
| Pressure (GPa) | Energies (Ry) | Volume (a.u.)3 | Energy gap (eV) | dSr–F (Ǻ) | dK–F (Ǻ) | dX–F (Ǻ) (X = Li, Na, K, Rb) |
||
|---|---|---|---|---|---|---|---|---|
| Present (GGA) | Present (mBJ) | Previous (0.00 GPa) | ||||||
| 0 | −8163.848 | 691.754 37 | 3.307 | 6.799 | 3.31 |
2.579 | 2.601 | dSr–F = 2.57 (0.00 GPa) |
| 5 | −8163.820 | 644.114 32 | 3.537 | 6.982 | 3.23 |
2.572 | 2.598 | dLi–F = 1.85 (0.00 GPa) |
| 10 | −8163.804 | 598.713 34 | 3.633 | 7.120 | 3.30 |
2.564 | 2.592 | dNa–F = 2.23 (0.00 GPa) |
| 15 | −8163.795 | 555.497 52 | 3.727 | 7.198 | 6.79 |
2.558 | 2.586 | dK–F = 2.60 (0.00 GPa) |
| 20 | −8163.782 | 514.412 99 | 3.755 | 7.235 | 2.546 | 2.581 | dRb–F = 2.74 (0.00 GPa) | |
| 25 | −8163.771 | 475.405 84 | 3.823 | 7.302 | 2.539 | 2.577 | ||
Figure 1. The pressure variation of lattice constant (a) GGA (b) LDA.
Download figure:
Standard image High-resolution imageFigure 2. The pressure variation of bonds length (a) Sr–F (b) K–F.
Download figure:
Standard image High-resolution imageFigure 3. The pressure dependence of band gap (a) GGA (b) mBj.
Download figure:
Standard image High-resolution imageFigure 4. The electronic band structures of SrKF3 under the application of pressure (0, 5, 10, 15, 20 and 25 GPa) calculated using GGA approximation.
Download figure:
Standard image High-resolution imageFigure 5. The total and partial density of states (TDOS & PDOS) of SrKF3 at 0 and 25 GPa using GGA approximation.
Download figure:
Standard image High-resolution image3.2. Elastic and mechanical properties
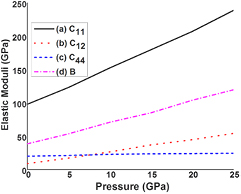
The mechanical stability of cubic crystals under pressure can be determined by elastic constants. In order to verify the change in behavior of mechanical parameters under pressure of (0-25GPa), we used to perform calculations at different values of reduced volumes, each of them corresponds to specific value of hydrostatic pressure. The complete optimization against each value of pressure is done with the step size of 5 GPa pressure. Furthermore, for computing stress tensor, we employ Charpin method [42]. For cubic system, there are three independent elastic constants namely C11, C12, and C44 respectively [43]. In this section, our main aim is to analyze effect of pressure variation on tensile strength, shear strength, rigidity, brittle/ductile behavior, and trends of directional/ non-directional bonding, average sound velocities, and Debye temperature in cubic SrKF3 fluoroperovskite compound. The details of all calculated elastic and mechanical parameters can be found in the following references [44–46]. Figure 6 presents the variation of elastic moduli such as C11, C12, and C44 and B with regard to different values of pressure. From figure 6 and table 3 it can be observed that all elastic constants increase with the rise in compression. However, a prominent increase is observed in C11 elastic constant, which is concerned with elasticity in length and bulk modulus, which is measure of hardness of the solid. The change in the value of C11 is from 98.101 GPa to 238.746 GPa and the change in bulk modulus value is from 38.71 GPa to 119.74 GPa respectively. The increase in C11 can be best explained in relation with bond strength enhancement (Sr–F, and K–F) because at elevated pressures there is decrease in the bond length and increase of charge density as mentioned in the previous sections. The elastic moduli of shape deformation explore less effect against variation in pressure,which indicates that enhancement of bond strength is less effected by shear deformation. To verify the stability of cubic SrKF3, we calculate elastic constants for strontium based fluoroperovskite as shown in table 4 within pressure range from 0–25 GPa. It is found that SrKF3 compound obey the modified stability criteria [47] for cubic crystals under finite strain till 20 GPa i.e.



Table 3. Calculated values of elastic constants (C11, C12, C44), Bulk modulus (B0), Voigt's shear modulus (GV), Reuss's shear modulus (GR) and Hill's shear modulus (GH), Young's modulus (Y), B/G ratio, Poisson's ratio (ѵ), Cauchy pressure (C''), Anisotropy constant (A), Kleinman parameter (ξ), and melting temperature (Tm), longitudinal (υl in m s−1), transverse (υt in m s−1), average sound velocity (υm in m s−1), Debye temperature (θD in K) of SrKF3 at pressure from 0–25 GPa.
| Pressure (GPa) | 0 | 5 | 10 | 15 | 20 | 25 | Previous work (0.00 GPa) |
|---|---|---|---|---|---|---|---|
| C11 (GPa) | 98.101 | 123.268 | 152.542 | 179.842 | 207.197 | 238.746 | 98.230 |
| C12 (GPa) | 9.021 | 17.289 | 26.598 | 37.128 | 44.895 | 54.385 | 9.019 |
| C44 (GPa) | 20.189 | 21.221 | 22.985 | 23.682 | 23.998 | 24.512 | 20.170 |
| Bo (GPa) | 38.71 | 53.71 | 71.12 | 85.26 | 104.07 | 119.74 | 38.123 |
| Gv (GPa) | 29.929 | 33.928 | 38.980 | 42.752 | 46.859 | 51.579 | 29.854 |
| GR (GPa) | 25.840 | 27.915 | 30.811 | 32.319 | 33.410 | 34.702 | 25.794 |
| GH (GPa) | 27.885 | 30.922 | 34.895 | 37.536 | 40.135 | 43.140 | 27.884 |
| Y (GPa) | 67.457 | 77.830 | 89.971 | 98.197 | 106.689 | 115.545 | 67.397 |
| B/G (GPa) | 1.388 | 1.737 | 2.038 | 2.271 | 2.593 | 2.776 | 1.375 |
| Ѵ (GPa) | 0.210 | 0.258 | 0.289 | 0.308 | 0.329 | 0.339 | 0.210 |
| C'' | −11.168 | −3.932 | 3.613 | 13.446 | 20.897 | 29.873 | −11.167 |
| A (GPa) | 0.453 | 0.400 | 0.365 | 0.332 | 0.296 | 0.266 | 0.451 |
| ξ (GPa) | 0.255 | 0.316 | 0.360 | 0.403 | 0.416 | 0.431 | 0.256 |
| Tm (K) | 1522.00 | 1661.50 | 1823.42 | 1954.92 | 2129.85 | 2275.58 | 1522.96 |
| υl (m s−1) | 2597.780 | 2733.618 | 2902.544 | 3007.808 | 3106.828 | 3217.591 | — |
| υt (m s−1) | 4285.592 | 4789.912 | 5329.490 | 5710.698 | 6156.192 | 6522.205 | — |
| υm (m s−1) | 3288.003 | 3520.575 | 3783.154 | 3951.142 | 4118.743 | 4284.748 | — |
| θD (K) | 396.592 | 442.814 | 481.950 | 518.056 | 549.218 | 578.968 | — |
aMubarak [14]. bMubarak and Mousa [7]. cErum and Iqbal [15].
Table 4. Derived elastic constants characterizing mechanical stability (equations (1)–(3)) of SrKF3 at pressure from 0–25 GPa.
| Pressure (GPa) | M1 | M2 | M3 |
|---|---|---|---|
| 0 | 38.71 | 20.19 | 44.54 |
| 5 | 54.28 | 16.22 | 47.99 |
| 10 | 71.91 | 12.99 | 52.97 |
| 15 | 89.70 | 8.68 | 56.36 |
| 20 | 105.66 | 4.00 | 61.15 |
| 25 | 124.17 | −0.49 | 67.18 |
Figure 6. Calculated pressure dependence of elastic constants (a) C11 (b) C12 (c) C44 (d) Bulk modulus, B for SrKF3 compound.
Download figure:
Standard image High-resolution imageIt can be noticed from figure 7 and table 4 that calculated elastic constants do not satisfy stability condition from equation (2) because at 25 GPa pressure stability criteria M2 (equation (2)) value is lower than zero, which implies that calculated elastic constants cannot satisfy all mechanical stability conditions, hence the compound is not mechanically stable above 20 GPa which is in agreement with our electronic structure calculations that the compound is mechanically stable against pressure less than 25 GPa. The Shear modulus (GH), and Young's modulus (Y) also increase with the elevated pressure in similar trend with the value of Bulk modulus (B). For example, the value of GV, GR, GH, and Y at 25 GPa is 51.579 GPa, 34.702 GPa, 43.140 GPa, and 115.545 GPa are several times greater than at 0 GPa respectively, indicating that SrKF3 compound becomes less compressible at higher pressures ranges. In addition, we observed that with the change in pressure from 0 to 25 GPa the transition is observed from brittle to ductile behavior because B/G value shift from 1.39 to 2.80 and it is previously reported in our own work [2] that, the B/G > 1.75 classify material as ductile, while B/G < 1.75 classify material as brittle. Similar effect of pressure variation is observed for poison's ratio (ѵ). The angular characteristics of atomic bonding via Cauchy's law or Cauchy pressure [48] demonstrates comparable transition from negative value of −11.68 to the positive value of 29.87 respectively as with the increase in pressure. But according to widening trend of bandgap this change in sign is just an indicator in reduction of its angular characteristics [49]. The similar kind of pressure induced behavior is observed for other fluoroperovskite of the same series such as BaLiF3 and SrLiF3 [30]. Next, we interpret value of elastic anisotropy factor (A), it can be noticed that the degree of deviation of anisotropic behavior is increased with the increase in pressure. The effect of varying pressure on bond bending trend, as given by Kleinman parameter [50, 51], agrees well with the above-mentioned responses. The trend of melting temperature depicts that an increase in pressure induces less tendency of melting extent of SrKF3 compound, which eventually increases its melting temperature.
Figure 7. Stability criteria for Cubic SrKF3 compound as a function of pressure.
Download figure:
Standard image High-resolution imageThe Debye temperature (θD) or Debye cut-off frequency is a significant form of temperature, which used to quantify several thermodynamic properties in the solid. It is basically a measure of the vibrational response of the crystal. In actual, it is the temperature above which the crystal behaves classically. There are two main methods to calculate Debye temperature (θD) including elastic constant method and specific heat measurement method. In this section, we consider elastic constant method to calculate Debye temperature (θD) and associated parameters following the formulas of the reference [52]. It can be observed from table 3 that wave velocities show a quadratic variation which increases monotonically with rising pressure. However, they can be divided into two groups because longitudinal wave velocity increases rapidly with pressure and is greater than the shear wave velocity. As Debye temperature is directly derived from the elastic wave velocity, so the similar trend of rise is also observed for Debye temperature (θD). This monotonic increase can be attributed to the increase in the bulk modulus under elevated pressure ranges because it is well known that the Debye temperature is proportional to the bulk modulus and increase in bulk modulus induces a phenomenon of phonon softening under pressure. As per best of our knowledge, there is lack of available experimental data to compare elastic wave velocity and Debye temperature under pressure, and we hope our study can provide useful guidance for future investigations.
3.3. Thermodynamic properties
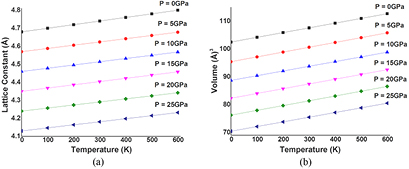
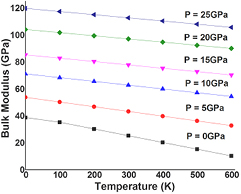
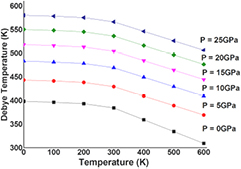
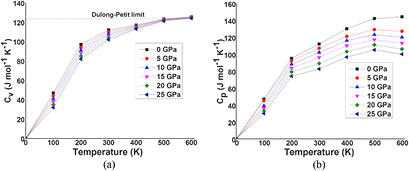
The thermodynamic properties of SrKF3 compound are used to obtained from Quasi-harmonic Debye model as implemented in the Gibbs program. The calculated details can be seen in the previously published work [25]. The pressure effects are determined in the range of 0–25 GPa, in which cubic stability of SrKF3 fluoroperovskite remains valid and thermal properties are studied in the temperature range from 0 to 600 K where Quasi-harmonic Debye model remains valid [51]. Figures 8(a) and (b) presents the relation between lattice parameters (lattice constant and unit cell volume) and figure 9 presents isothermal bulk modulus with temperature T up to 600 K at P = 0 GPa, 5 GPa, 10 GPa, 15 GPa, 20 GPa, and 25 GPa respectively. It can be noticed that both temperature and pressure have inverse relation with lattice parameters and bulk modulus because at a given pressure lattice parameters such as lattice constants and volume expansion increases with the increasing temperature and bulk modulus decreases with increasing temperature at a given pressure and vice-versa. In fact, at higher pressure thermal effects becomes weaker and stronger for lattice parameters and bulk modulus respectively, which reveals that effect of pressure and temperature are inversely proportional to each other. So, increase in temperature causes a significant reduction in the hardness of SrKF3. Next, we explore as important fundamental parameter of Debye temperature θD that depicts many physical properties such as specific heat, elastic constants and melting extent of a material [53]. From the plot of Debye temperature θD as shown in figure 10. It should be noted that the static values of the Debye temperature (at T = 0 and P = 0) calculated from the quasi-harmonic Debye model is 396.989 K which is very close to the value computed from elastic properties (396.512) as listed in table 3. However, to the best of our knowledge there is no experimental data for comparison with our calculated values. One can notice that (a) for a fixed pressure, θD decreases with increase in temperature and for a fixed temperature, θD increases with the increasing pressure. (b) The process of increase in pressure and decrease in extent of melting temperature will lead to increase Debye temperature (θD) and bulk modulus because at higher pressure ranges bulk modulus increases, as a result a phenomenon of phonon softening takes place and θD will increase in accordance with previous temperature ranges [54]. At the end, we analyze material's extent to store heat in terms of specific heat capacities at constant volume and constant pressure CV and CP respectively. It can be observed from figures 11(a) and (b) that variation of CV and CP are similar to each other as temperature increases. However, the trend of CV increases slowly following a common phenomenon in all solids at higher temperature ranges by approaching towards Dulong–Petit limit (123.7 J mol−1 K−1) [55] where anharmonic effects prominently suppressed. However, CP behavior deviates from T > 300 K and does not converge to a constant value. In particular, when pressure vanishes, the values of CP increases rapidly at about higher temperature. Unfortunately, there is lack of experimental data on the thermodynamic properties to compare with but we are in hope that our work will serve as a reference material for other co-researchers of this filed.
Figure 8. (a) Variation of the Lattice constant as a function of temperature at different pressures for SrKF3 compound. Figure (b) variation of the unit cell volume as a function oftemperature at different pressures for SrKF3 compound.
Download figure:
Standard image High-resolution imageFigure 9. Variation of the Bulk modulus as a function of temperature at different pressures for SrKF3 compound.
Download figure:
Standard image High-resolution imageFigure 10. Variation of the Debye temperature (θD) as a function of temperature at different pressures for SrKF3 compound.
Download figure:
Standard image High-resolution imageFigure 11. (a) Variation of the specific heat capacities of Cv as a function of temperature at different pressures for SrKF3 compound. Figure (b) variation of the specific heat capacities of Cp as a function of temperature at different pressures for SrKF3 compound.
Download figure:
Standard image High-resolution image4. Conclusions
Effect of pressure variation on detailed physical properties are investigated for the first time by ab-inito DFT method for cubic phase of SrKF3 fluoroperovskite compound. The calculated equilibrium lattice parameters are in good agreement with previous theoretical and experimental reports at 0 GPa. It is observed that an increase in pressure considerably improves the wide and direct (Γ–Γ) electronic nature of bandgap because at elevated pressure ranges bands broadened the energy of Sr-3d and Sr-4d states thereby resulting in an increase in the ratio of splitting between Sr-4d, Sr-3d, K-2p, F-2s and F-2p states which ultimately results an increase in the bandgap of the material. The pressure dependence of elastic constants and significant mechanical parameters confirm compound's mechanically stability in cubic structure till 20 GPa. Moreover, an increase in pressure improves tensile strength and stiffness, on the other hand, reduces brittleness and compressibility of the SrKF3 compound. The effect of thermodynamic parameters on macroscopic properties are predicted to utilize this material in temperature dependent applications implementing Quasi-harmonic Debye model within the range 0–25 GPa and 0–600 K with the step size of 5 GPa and 100 K respectively. Consequently, we believe that our work will motivate research scholars to done theoretical as well as experimental studies in this direction, which must be taken into account to understand and utilize this material in fabricating practical devices.