Abstract
Silver nanoparticles (Ag NP) have been used for over a century for many purposes including as germicides. Unique physical, chemical and biological properties of Ag NPs make them suitable for a wide range of applications in different industries and biomedical fields. Green nanobiotechnology with synthesis of NPs using biomolecules (protein, enzyme, DNA and plant extracts) have become a rapidly developing research area. Green synthesis methods have overcome the disadvantages of traditional physical and chemical synthesis approaches, such as high cost, long time scales and toxicity. In the green route, the biomolecules act as both reducing and/or stabilizing agents to produce biocompatible NPs. Promising results on antimicrobial activity of Ag NP against several pathogenic microorganisms have been reported in literature. The growth of multiple antibiotic resistant bacteria could be inhibited by using Ag NP. This review mainly discusses the synthesis routes and characterization of biomolecules capped Ag NPs and their enhanced antimicrobial properties towards various human and plant pathogens.
Export citation and abstract BibTeX RIS
1. Introduction
Nanotechnology is the science of modulating metals into particles ranging at sizes between 1 and 100 nm [1–3]. At these sizes, the properties of nanoparticles (NPs) are different from those of the bulk. Scientists extensively studied metallic NPs for their applications in engineering and medical fields. Silver nanoparticles (Ag NPs) are particularly important due to their unique and intrinsic physical, chemical and biological properties, those of which open up new avenues for their applications in various scientific and industrial fields [4, 5]. For instance, optical and catalytic properties of Ag NPs are highly shape and size dependent and which make Ag NPs use in biomedical areas [6]. In terms of industrial use of Ag NPs, they have been in used as safe preservatives in different cosmetic products due to non-penetrating property to human skin [7, 8]. In agriculture, Ag NPs have been also used to control an important crop pest and diseases towards Eobania vermiculata (Land Snail), Xanthomonas perforans and Fusarium graminearum (cause head blight of wheat), Fusarium oxysporum (cause tomato wilt), Fusarium solani (cause potato rot), and Penicillium expansum (cause apple rot) [9–13]. In addition to those applications, Ag NPs play a pivotal role in removal of pesticide contamination by mineralization [14]. Ag NPs have significant antibacterial effect against Gram- positive and Gram- negative bacteria like Bacillus subtilis, Staphylococcus aureus Pseudomonas aeruginosa, Klebsiella pneumonia and Shigella flexneri [15–18]. Additionally, they have been used as antiviral (by the shipment of HIV −1 in human cervix organ in culture media), anti-inflammatory and anticancer agents [19, 20].
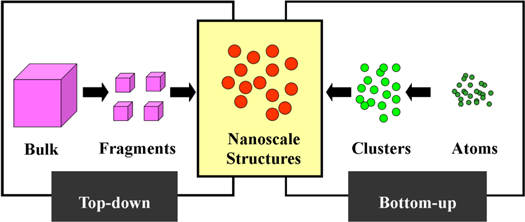
In synthesis of nanomaterials, two major processes have been commonly used. First one is top-down approach which relies on breaking down a bulk material via different techniques like sputtering, chemical etching, thermal/laser ablation, and mechanical/ ball milling and explosion process to obtain nano sized ones (figure 1) [21]. Second one is bottom-up approach in which atoms of elements or precursors assembles through condensation, vapor deposition, sol-gel process, spray pyrolysis, chemical/electrochemical deposition, aerosol methods or bioreduction processes to form NPs of the desired material [5].
Figure 1. Top- down and bottom- up approaches in nanotechnology.
Download figure:
Standard image High-resolution imageTherefore, top-down and bottom-up approaches include physical, chemical and biological methods as major methods for the preparation of nanomaterials [22]. In the chemical reduction method, Ethylene Glycol (EG) is used as the reducing agent whereas Polyvinyl Pyrrolidone (PVP) stabilizes the Ag NPs. The size of synthesized nanoparticles depends on the pH of solution [23]. Poly Ethylene Glycol (PEG) is also used as a capping agent to inhibit aggregation [24]. In an alternative chemical reduction method silver nitrate (AgNO3) is reduced by sodium borohydride (NaBH4) [25]. Silver colloid is also prepared using trisodium citrate as reducing agent [26]. Mechanism of chemical reduction is shown in equation (1). Mechanisms of Chemical Reduction. [18]
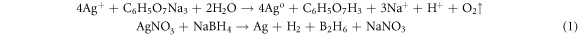
The physical method includes several techniques like laser ablation, gamma radiation, electron radiation or microwave processing [27]. In a study NPs are prepared by γ-irradiation at various doses (25, 35, and 45 kGy) [28].
The physical methods are considered expensive and time consuming. The chemicals used in chemical reduction like Sodium borohydride, thio-glycerol and 2-mercaptoethanol are hazardous to environment [29]. Green chemistry provides alternative options to overcome some of these problems and the green route of synthesis of Ag NPs is cost effective and eco-friendly [30]. Use of biomolecules in the synthesis of Ag NPs is a novel method for scaled up synthesis. Microorganisms and plant extract have been used widely for the synthesis of metallic nanoparticles [31, 32]. Abundance of raw materials (Bioreductant) is an important advantage in the green synthesis [33]. Several reviews on the green synthesis of Ag NPs have been published in last few years. The goal of this review is to summarize the synthesis procedures of Ag NPs from the cost efficiency and eco-friendliness point of views. Only plant extract mediated synthesis route is considered here. Characterization and antimicrobial properties of Ag NPs are also mentioned in this article.
Biomolecules mediated of silver nanoparticles (Ag NPs)
Several biomolecules including DNA, protein, enzyme, peptide, amino acids and plant extracts, have been employed in biosynthesis of various NPs, especially Ag NPs [11–13, 34–41]. Those molecules act as nucleation template, reducing and capping agents for in situ production of biocompatible Ag NPs in aqueous solutions. This approach overcomes the drawbacks of chemical synthesis methods mentioned previously. It also removes phase transfer steps for NPs synthesized in organic media.
DNA metallization has received great attention owing to its unique scaffold function to form inorganic metallic NPs. The Watson-Crick base pairing provides a DNA programmable structure to control the nucleation, growth and completion of NPs and as well as positioning [42–47]. In general, DNA consist of two major components, nucleobase (adenine (A), thymine (T), guanine (G), cytosine (C)), and phosphate backbone. Each component holds different chemical groups for binding of metal ions. For instance, the bases of DNA interact with mono-and divalent metal ions (Ag+, Hg2+, Pt2+) which promotes the synthesis of those NPs, especially synthesis of Ag NPs [11–13, 42, 43]. Various formations and positioning of DNA-NPs are presented in figure 2.
Figure 2. The illustraiton of DNA sequence and NPs interaction.
Download figure:
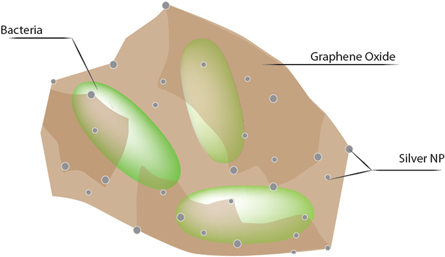
Standard image High-resolution imageIn a previous study, Ocsoy and coworkers produced double stranded DNA (dsDNA) templated Ag NP and Ag NP decorated graphene oxide (GO) nanocomposites for controlling the bacterial spots caused by Xanthomonas perforans on tomatoes plant [11–13]. They designed the dsDNA with eight base pairs extention tails to adsorb it on the GO surface through π-π stacking. The major groove of dsDNA acts as a nest for binding of Ag+ ions and Ag NPs were formed in the presence of reducing agents. The Ag@GO nanocomposites attack the target bacteria and wrap them as in swaddling to effectively inactivate them. The wrapping process between bacterial cells and Ag@GO nanocomposite is illustrated in figure 3.
Figure 3. Ag@GO nanocomposite adheres and wraps the bacterial cells for rapid and effective killing.
Download figure:
Standard image High-resolution imageIn another study by Wu et al, morphological evolution Ag NPs was studied using different DNA sequences. The types and length of oligo may play a vital role in terms of DNA programmable structure in interaction of oligo nucleotides and Ag+/Ag NPs. It is claimed that 10-base long oligo-nucleotides, adenine (A) and thymine (T) results in differently truncated satellite octahedral shaped Ag NPs and cytosine (C) induce truncated tetrahedral. In contrast to them, original cubic shape and size Ag NPs grown from cubic seeds remain with base long guanine (G). The Ag NPs with various shapes offer morphology and size dependent optical properties plant [42].
In addition to DNA based Ag NP formation, amino acids, protein and enzyme molecules directed various sized and shaped Ag NP have been produced. For instance, free form of tyrosine, tryptophan and aniline amino acids and glutamic acid embeded gelatine polypeptide, acted as reducing and stabilizing agents to synthesize the uniform and mono-dispersed spherical Ag NPs under various experimental conditions [34–39]. While the etratricopeptide repeat (CTPR) proteins form triangular Ag nanoprisms with enhanced optical property, two proteins with different molecular weight and structures, bovine serum albumin (BSA) and lysine (Lyz) produce prism and spherical shaped Ag NPs based on their capping model on Ag seed [38, 39]. The Ag NPs have been also synthesized by using various enzymes with their different activity mechanism.
Ag NPs with various morphologies were also synthesized using enzymes acted as reducing and capping agents. For instance, Macerating enzymes was used to reduce Ag+ ions for formation of Ag NPs in vitro system. Additionally, the simultaneous addition of horseradish peroxidase for catalysis of reducing of Ag+ ions and silver enhancement kit (EnzMetTM) provides rapid formation of Ag NPs. We may conclude that enzymatic reaction offers an environment-friendly, simple, rapid, one step and easy process for synthesis of Ag NPs even in aqueous solutions or on solid substrates.
Plant extract mediated green synthesis of silver nanoparticles (Ag NPs)
Although DNA, protein, enzyme, peptide and amino acid have been safely and rationally benefited for biosynthesis of Ag NPs. Those biomolecules carry several disadvantages including their high cost, easy contamination risk and slow stability toward experimental conditions. Among biomolecules, plant extracts have great advantages over others in all metallic NPs. The plant extracts are quite cheap, easy available, almost no contamination risk and very stable against harsh environmental conditions (high, temperatures, wide range of pH and salt concentrations).
In the synthesis of Ag nanoparticles, green route is considered as economic, nontoxic, faster, one step and environmentally safe procedure. Biogenic Ag NPs are synthesized using various plant parts extract like leaves, fruit, seed, bark etc [48–59]. Biomolecules perform as reducing as well as stabilizing agent in green synthesis. So, there is no need to add hazardous agents from outside [60]. Several plant metabolites like terpenoids, polyphenols, sugars, alkaloids, phenolic acids, and proteins, play a crucial role in the bioreduction of silver (Ag+) [61]. Several works have been published regarding synthesis of Ag NP using different plant extract [62]. Medicinal plants have got importance by the synthesis method due to presence of alkaloids, terpenoids, flavonoids and phenolic compounds etc [63]. The diversity of plants used in green synthesis of Ag NPs has mentioned in table 1. Nanoparticles are synthesized with in very short time at room temperature.
Table 1. Plans Used in Green synthesis of Silver Nanoparticles.
| Sl No. | Name of Plants | Used Parts | Size(nm) | Shape | Author(s) |
|---|---|---|---|---|---|
| 1 | Achillea biebersteinii | Flower | 12 ± 2 | — | [64] |
| 2 | Alstonia scholaris | Leaf | — | — | [65] |
| 3 | Alternanthera dentata | Leaf | — | — | [66] |
| 4 | Azadirachta indica | Leaf | 34 | Spherical | [63] |
| 5 | Camellia sinensis | Leaf | 4 | Cubic | [67] |
| 6 | Carica papaya | Leaf | 50–250 | Spherical | [68] |
| 7 | Catharanthus roseus | Leaf | 35–55 | Cubic | [69] |
| 8 | Cicer arietinum | Leaf | 88.8 | Spherical | [70] |
| 9 | Cocos nucifera | Inflorescence | 22 | — | [71] |
| 10 | Cornus officinalis | Fruit | 11.7 | Quasi-Spherical | [72] |
| 11 | Croton bonplandianum | Leaf | 17.4 | Spherical | [60] |
| 12 | Delphinium denudatum | Root | 85 | Spherical | [73] |
| 13 | Detarium microcarpum | Leaf | 17.05 | Spherical | [74] |
| 14 | Emblica officinalis | Fruit | 15 | Spherical | [75] |
| 15 | Eucalyptuschapmaniana | Leaf | — | Cubic | [76] |
| 16 | Ficus benghalensis | Prop root | 42.7, 51.4 | Spherical | [77] |
| 17 | Hemidesmus indicus | Leaf | 25.24 | Spherical | [78] |
| 18 | Holarrhenaantidysenterica | Bark | 32 | Spherical | [79] |
| 19 | Lantana camara | Leaf | — | Spherical | [80] |
| 20 | Mimosa pudica | Seed | 40 | — | [81] |
| 21 | Mimusops elengi | Leaf | 55–83 | Spherical | [82] |
| 22 | Nelumbo nucifera | Seed | 5.03–16.62 | Spherical | [83] |
| 23 | Ocimum sanctum | Leaf | 42 | Triangle | [84] |
| 24 | Phyllanthus amarus | Leaf | 15.7, 24 ± 8, 29.78 | — | [85] |
| 25 | Pinus eldarica | Bark | 10–40 | Spherical | [86] |
| 26 | Piper nigrum | Leaf and Stem | 7–50; 9–30 | — | [87] |
| 27 | Plectranthusamboinicus | Leaf | — | Spherical | [88] |
| 28 | Pongamia pinnata | Seed | 16.4 | Spherical | [89] |
| 29 | Sesbania grandiflora | Leaf | 10–25 | Spherical | [90] |
| 30 | Solanum xanthocarpum | Fruit | 10 | Spherical | [91] |
| 31 | Trifolium resupinatum | Seed | 17 | Spherical | [92] |
| 32 | Viburnum opulus | Leaf | 25 | Spherical | [93] |
| 33 | Zea mays | Silky Hairs | 249.12 | — | [94] |
| 34 | Zingiber officinale | Rhizome | 10–20 | — | [95] |
| 35 | Ziziphora tenuior | Leaf | 8–40 | Spherical | [96] |
In an experiment leaves and fruits of Solanum nigrum are dried to form fine powder. Plant extract is produced from 10 g of powder, is added to 200 mL double distilled water followed by shaking for 30 min. Then it is kept in boiling bath for 10 min [97]. In another study leaf extract is produced from Azadirachta indica. The extract is produced from 20 g leaves, are boiling in 200 mL double distilled water for 30 min [63]. Beg et al formulated the preparation of leaf extract of Croton bonplandianum, by boiling of 15 g leaves in 150 ml deionized water at 95 °C for 45 min [60]. Filtered extract is now added to Silver nitrate solution for synthesis.
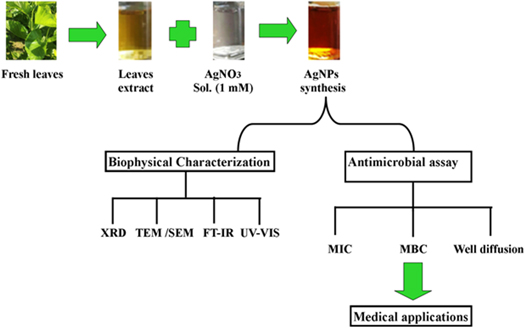
The experiment is performed at room temperature in a dark chamber to minimize the photo reaction [63]. Sometimes alcohol mediated plant extract is used for synthesis [91]. AgNPs are produced from standard concentration of silver nitrate (1 mM AgNO3). Sometime different concentration (1–5 mM) of AgNO3 is used for optimization of reaction. It is found in an experiment that AgNPs are produced from a reaction mixture of 10 ml AgNO3 and 4 ml leaf extract of Azadirachta indica. Reduction process is completed by color change (colorless to brown) of the solution. Reaction is confirmed by UV–vis Spectroscopy. Figure 4 present the synthesis of plant extracts directed Ag NP.
Figure 4. Plant extract mediated synthesis of silver nanoparticles.
Download figure:
Standard image High-resolution imageIt is reported that green synthesis of AgNP is performed from 1 mM aqueous AgNO3 using 10% leaf extract of Alstonia scholaris [65]. The nanoparticles are purified by centrifugation process. Supernatant is discarded and purified AgNP is resuspended in deionized water and used for characterization [89]. Plant Extracted mediated synthesis of Ag NPs is focused in Scheme 3.
Characterization of silver nanoparticles
The nanoparticles are characterized by different instrumental techniques like UV–visible Spectroscopy, Atom Force Microscopy (AFM), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Energy Dispersive x-ray (EDX) Spectroscopy, x-ray Diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR) and Zeta Laser Doppler Electrophoresis [89–96, 98]. The primary characterization of nanoparticles has been documented by UV- Vis spectroscopic method [99]. Surface Plasmon Resonance (SPR) is calculated by absorption peak for presumptive analysis of particle size [100]. The maximum absorbance data of Ag NPs range between 350–500 nm. It is published that the maximum absorbance at 425 nm is recorded in mushroom extracted glucan mediated synthesis of AgNP [67]. In another investigation λmax is recorded at 430 nm for Neem-AgNPs [101]. Fourier Transform Infrared Spectroscopy (FTIR) is an analytical technique to identify the functional groups responsible for bio reduction of Ag+. It is reported that the functional groups like O–H, N–H,  CONH2 and COOH are involved in Croton bonplandianum leaves extract mediated green synthesis of AgNPs [60]. Tho et al, reported that amine, aromatic and alkynes groups are responsible for reduction in Nelumbo nucifera seed extract mediated synthesis [84]. Glucoxylans in seed extract of Mimosa pudica act both as reducing and capping agents [82]. Polyphenols, glucose and fructose are wonderful capping chemicals in fruit of Emblica officinalis. The capping agents like glucose, fructose etc reduce the toxicity of AgNP. Various morphological features and parameters of nanoparticles are recorded by AFM [75]. The morphology of nanoparticles is determined by electron microscopy. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are two important instrumental techniques to measure the shape and size of nanoparticles [102]. Elemental composition of nanoparticles is confirmed by Energy Dispersive x-rays (EDX). High negative zeta potential indicates the repulsion and dispersion stability of nanoparticles [103]. The synthesized Ag NPs are showing −55.0 mV zeta value, reported by Subba Rao et al [84]. X-ray Diffraction (XRD) analysis is analytical tool to study the nature of AgNPs. In a synthesis procedure, Ag NPs are characterized as crystalline in nature with face centered cubic (fcc) shape [104]. Well dispersed and face centered nature of Ag NPs are also characterized by Velmurugan et al [95].
CONH2 and COOH are involved in Croton bonplandianum leaves extract mediated green synthesis of AgNPs [60]. Tho et al, reported that amine, aromatic and alkynes groups are responsible for reduction in Nelumbo nucifera seed extract mediated synthesis [84]. Glucoxylans in seed extract of Mimosa pudica act both as reducing and capping agents [82]. Polyphenols, glucose and fructose are wonderful capping chemicals in fruit of Emblica officinalis. The capping agents like glucose, fructose etc reduce the toxicity of AgNP. Various morphological features and parameters of nanoparticles are recorded by AFM [75]. The morphology of nanoparticles is determined by electron microscopy. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are two important instrumental techniques to measure the shape and size of nanoparticles [102]. Elemental composition of nanoparticles is confirmed by Energy Dispersive x-rays (EDX). High negative zeta potential indicates the repulsion and dispersion stability of nanoparticles [103]. The synthesized Ag NPs are showing −55.0 mV zeta value, reported by Subba Rao et al [84]. X-ray Diffraction (XRD) analysis is analytical tool to study the nature of AgNPs. In a synthesis procedure, Ag NPs are characterized as crystalline in nature with face centered cubic (fcc) shape [104]. Well dispersed and face centered nature of Ag NPs are also characterized by Velmurugan et al [95].
Antimicrobial activity of silver nanoparticles
The Ag NPs are wonderful bioactive compounds for its antimicrobial potency [102, 105, 102–104, 106]. Silver and its compounds are widely used for the treatment of bacterial infections like wounds and burns [107]. It has been recognized as non-toxic and safe therapeutic drug in medical sciences [33]. The particles showed excellent antimicrobial activity against Gram+ and Gram–bacteria [75]. Several studies have been established to prove the antimicrobial potency of Ag NPs (table 2).
Table 2. Antimicrobial activities of silver nanoparticles.
| Sl No. | Biogenic Source(s) | Size (nm) | Tested Bacteria | Author(s) |
|---|---|---|---|---|
| 1 | Agave americana | 30–150 | Escherichia coli and Staphylococcus aureus (MRSA) | [63] |
| 2 | Alstonia scholaris | — | Escherichia coli ATCC 25922 | [65] |
| 3 | Alternanthera dentata | — | Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia and Enterococcus faecalis | [66] |
| 4 | Cicer arietinum | 88.8 | Escherichia coli and Pseudomonas aeruginosa | [70] |
| 5 | Cocos nucifera | 22 | Klebsiella pneumonia, Bacillus subtilis, Pseudomonas aeruginosa and Salmonella paratyphi | [71] |
| 6 | Croton bonplandianum | 17.4 | Escherichia coli ATCC 25922 | [60] |
| 7 | Delphinium denudatum | 85 | Staphylococcus aureus ATCC 6538, Bacillus cereus NCIM 2106, Escherichia coli ATCC 8739 and Pseudomonas aeruginosa ATCC 9027 | [73] |
| 8 | Ficus benghalensis | 42.7, 51.4 | Streptococcus mutans and Lactobacilli spp. | [77] |
| 9 | Hemidesmus indicus | 25.24 | Shigella sonnei | [78] |
| 10 | Lantana camara | — | Escherichia coli, Pseudomonas spp., Bacillus spp. and Staphylococcus spp. | [80] |
| 11 | Phyllanthus amarus | 15.7, 24 ± 8, 29.78 | Pseudomonas aeruginosa | [85] |
| 12 | Plectranthus amboinicus | — | Escherichia coli | [88] |
| 13 | Sesbania grandiflora | 10–25 | Salmonella enterica and Staphylococcus aureus | [90] |
| 14 | Solanum xanthocarpum | 10 | Helicobacter pylori | [91] |
Literature revealed the antibacterial activity of Ag NPs against multi drug resistant Gram + and Gram– bacteria [73–90]. Remarkable antibacterial activity is also proved against multi drug resistant Pseudomonas aeruginosa [85]. In a separate study Sen et al and Manna et al reported antimicrobial activity of glucan capped Ag NPs against multiple antibiotic resistant enteric bacteria respectively [101, 104]. The growth of food and clinical pathogens are inhibited by synthesized Ag NPs [95].
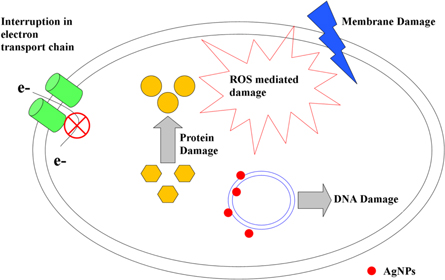
Antimicrobial assay on different bacteria is performed by Kirby-Bauer disc diffusion method [74, 107, 108]. Sometimes it is also performed by well diffusion method [59]. Ahmed et al used MacConkey broth for the culture of Escherichia coli and Staphylococcus aureus [63]. Antimicrobial potency of the synthesized Ag NPs was determined on MacConkey agar. The antimicrobial property of the plant extract used for the synthesis of AgNPs was also determined. Beg et al, reported that, 1.5 nM concentrations of Ag NPs synthesized via Croton bonplandianum leaf extract is recorded as minimum inhibitory concentration (MIC) against Escherichia coli ATCC 25922 [60]. Whereas, the MIC value of another AgNPs synthesized byAlstonia scholarisleaf extract was found to be 0.08 nM [65]. In a different study the MIC value is recorded 0.0015 mM against Bacillus cereus [109]. The AgNPs also showed remarkable antimicrobial potency against silk pathogens. The growth of gut bacteria of infected larvae of Bombyx mori is gradually decreased after feeding with Ag NPs treated mulberry leaves when compared with normal mulberry leaves [110]. Ag NPs antagonize against microbes by different mechanisms like adhesion, penetration, generation of reactive oxygen species, free radical and alter the signal transduction pathway [111]. Proposed mechanism of bacterial death induced by Ag NPs (figure 5).
Figure 5. Antimicrobials action of silver nanoparticles.
Download figure:
Standard image High-resolution imageAnju and Sarada reported that the Ag NPs exhibit quoroum sensing inhibition when checked against Chromobacterium violaceumvia inhibiting the violacein production [112]. In a different study, the virulence property of Pseudomonas aeruginosa is mediated by quorum sensing mechanisms of las and rhl system. The Ag NPs showed as anti-quorum sensing agent against this bacterium [113]. Thus, Ag NPs have the potential to alter the molecular mechanisms of microorganisms.
The Ag NPs showed promising antifungal activity on human pathogens. It is reported that 12.5 ± 4.9 nm Ag NPs are highly active against Candida albicans and C. tropicalis [114]. In a separate study the antifungal activity is proved against plant pathogenic fungi Rhizoctonia solani and Neofusicoccum parvum [98]. Ag NPs have found various applications in dentistry, especially in endodontics for deactivation of endodontic pathogens and destruction of biofilms [115–120]. Ag NPs as universal germicidal and Sodium hypochloride (NaOCl) recognised as an antibacterial have been utilized together to easily penetrate into the dentin tubules and induce deeper disinfection [115, 116]. AgNPs have been the most widely investigated in endodontic infections. AgNPs have ability to attach and penetrate into the cell walls of both Gram positive and Gram negative bacteria, they release silver ions and disturb cell function. Therefore, they are effective in treatment of drug-resistant microorganisms and they prevent and inhibit formation of biofilm [117]. Afkhami et al investigated antimicrobial property of AgNPs, in combination with photodynamic disinfection with diode laser against E. faecalis biofilm and suggested use of AgNPs and photodynamic therapy as an adjunct treatment to NaOCl [118]. In another in vitro study, Martinez–Andrade et al, and Rodrigues et al, used AgNPs in combination with 17% EDTA and 2% chlorhexidine, respectively towards in S. aerus and C. albicans and E. faecalis biofilm and found that AgNp had significantly more ability to dissolve biofilm compared with 17% EDTA and 2% chlorhexidine at 5 and 15 min [119, 120].
Conclusion
Green nano-biotechnology is an alternative to conventional physical and chemical synthesis of Ag NPs. Plants have been used in green route for its great diversity. Biomolecules present in plant extract act as both reducing as well as capping agent. Medicinal plants have great importance due to presence of alkaloids, flavonoids and other organic compounds. The goal of cost effectiveness and eco-friendly nature is fulfilled by this method. Synthesis of metallic nanoparticles plays a pivotal role in the field of biomedical sector. Ag NPs are more active against microbes. It has promising antimicrobial activity against several bacteria and fungi. Bacteria are being resistant to antibiotics due to its indiscriminate use. Gradually single to multiple antibiotics lose their fruitfulness to pathogenic bacteria. Therefore, use of Ag NPs is a new arsenal to combat against pathogens. Several papers are compiled in this review to find out the plant extract mediated synthesis of Ag NPs. Harmful protocol of synthesis is easily overcome this method. More research is urgently needed to define the exact mode of action of Ag NPs on microbes. Hope this review will be a new mile stone in future nano-biotechnological research to synthesize Ag NPs in green route.