Abstract
We reported an all single-mode fiber (SMF)-based polarization sensitive spectral domain optical coherence tomography (PS-SDOCT) system with single input polarization state (IPS). In our design, polarization state was modulated only by four polarization controllers (PC) and a polarization beam splitter. To extract the phase retardance and optical axis orientation of sample, the Mueller matrix of the fiber system and sample is transferred to a new retardar by employing PCs with the advantage that the effects of fiber birefringence of SMFs and SMF components are corrected. The contribution of the multi-backscattering in tissues to the orthogonal polarization interference signals is readily suppressed by subtracting the unpolarized component of the scattered light. To demonstrate the capabilities of our system, the crossing-sectional images were presented of phase retardance and optical axis orientation of several tissues. The experiment results showed that the imaging depth, the image contrast of tissues and signal-to-noise ratio have been further increased.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Corrections were made to this article on 30 January 2019. Corrections were made to the title.
1. Introduction
Optical coherence tomography (OCT) is a promising imaging technique that can provide high resolution cross-sectional images of sample architectures non-invasively [1]. In Fourier domain OCT (FDOCT), including spectral-domain OCT (SDOCT) and swept-source systems (SSOCT), the depth-resolved information is obtained by Fourier transforming the acquired interference spectral data [2–4]. However, conventional FDOCT can only generate intensity-based cross-sectional images of the microstructures of tissues. Polarization-sensitive optical coherence tomography (PS-OCT) is a functional extension of intensity-based OCT that utilizes the polarization properties of back-reflected light to obtain additional physiological information [5, 6]. Previous studies of PS-OCT have shown that PS-OCT has the capability of detecting fibrous tissues, including collagen fibers in skin, retinal nerve fiber layer, retardance imaging in carious lesions and oral tissues [7–13].
In the first PS-OCT system reported by Hee et al double detectors were used to measure the two orthogonal components of the light backscattered from the tissues [5]. For PS-OCT systems based on bulk-optics or polarization maintaining fiber (PMF) [14–16], the depth-resolved polarization information of the sample could be obtained without the influence of any unknown polarizing devices [14]. Wang et al proposed a PS-SDOCT system to measure reflectivity and retardance information of samples based on the use of PMF and a single line-scan camera [16]. However, due to the large volume of free space system, its clinical applications have been limited. In addition, cross coupling between the polarization channels at fiber connectors and splices can introduce ghost images and other artifacts in PMF-based system, degrading the image quality. On the other hand, PS-OCT based on single-mode fiber (SMF) has the advantages of easy alignment, no ghost peaks, and is possible for handheld probing [17]. Nevertheless, in the SMF-based system, SMFs will introduce unknown fiber birefringence to sample polarization information due to fiber imperfections [18].
Recently, several SMF-based PS-OCT methods have been proposed to remove the fiber disturbances, which can be subdivided into two categories by the number of input polarization states (IPS): multi-IPS and single IPS systems. In the multi-IPS systems, SMFs are allowed to be used by discrete modulation with a liquid crystal polarization rotator in the conventional sample arm [19], continuous modulation [20], depth multiplexing using a passive polarization delay unit [21–23] or frequency shifting [24, 25]. Although these methods circumvent the need for extracting the accurate polarization properties at the sample, they required additional modulation or polarization delay-line unit which may increase the cost and the complexity of system, or reduce acquisition speed. SMF-based PS-OCT implementations with single IPS offered easy alignment, low system complexity, low cost, no cross coupling, compact size, and the capability to be used for clinical diagnosis [26–29]. Although an all SMF-based PS-OCT (PSOCT1300, Thorlabs) [28] was proposed to provide polarization contrast imaging of tissues, there was no evidence that the system had a role in elimination the fiber disturbances to optical axis orientation map in biomedical tissues. Other methods have been demonstrated that the polarization information induced by the sample was extracted in PS-SSOCT systems by employing polarization controller (PC) [26, 27]. In addition, all reported fiber-based systems always included at least one linear polarizer, which decreased the measured signal and sensitivity of the systems.
In OCT, determination of the tissue scattering coefficient and quantitative depth ranging are generally based on single backscattering approximation [30]. However, for imaging of highly scattering media, multiple scattered photons are also present in the OCT signals due to the finite collection numerical aperture of the objective lens in the sample arm and coherence of the multiply scattered light. Wang et al have quantitatively showed that multiply scattered light could degrade signal and localization, reduces the imaging depth, and affect the image contrast [31]. Kalkman et al reported a method in which the effects of multiple scattering in Doppler OCT system could be separated and quantified [32].
In this paper, we report an all SMF-based polarization sensitive SDOCT system in which polarization state could be changed by four PCs and a polarization beam splitter (PBS). Based on Mueller matrix analysis, the fiber system and sample could be transferred to a new retardar by employing PCs, by which the phase retardance and optical axis orientation of sample could be extracted. In this case, the effects of fiber birefringence of SMFs and SMF components were corrected. Output Stokes vector of circular polarized light from the sample surface and equal reference illumination power at the detector outputs were provided by PCs, which were key elements to the proper functionality of the system. In order to improve the image quality, the contribution due to light multiply backscattered from tissues is removed by subtracting the unpolarized component of the imaging signals [33]. Images of ex vivo pork catilage, human finger nail and fingertip skin were presented which demonstrated the capabilities of extracting the phase retardance and optical axis orientation of tissues of our system. It was shown that the SNR in the cross-sectional image of human fingertip skin was increased by at least 2.6 dB.
2. Theory
2.1. Experimental setup
Our SMF-based OCT system is shown in figure 1 which is based on the Michelson interferometer. Figures 1(a) and (b) show the schematic and prototype of the handheld probe, respectively. The source used in our system was a superluminescent diode (SLD) with a center wavelength of 840 nm and a spectral bandwidth of approximately 50 nm. Thus an axial resolution of the system is 6.2 μm in air. The maximum power of the laser source was 54 mW. The light from SLD was guided into one port of the 2 × 2 fiber coupler without any polarizing components, and split equally into the reference and the sample arms of interferometer. In the reference arm, the light was reflected from a reference mirror. In the sample arm, a handheld probe was implemented with a galvanoscanner mirror for imaging tissues. The light backscattered from within the sample in the sample arm and the light reflected from the reference mirror were recombined at the fiber coupler and the interferogram generated was measured by a home-made spectrometer. The interference signal was then collimated by focusing lens with a focal length of 50 mm, dispersed by a transmission grating of 1200 lines/mm, focused by an achromatic focusing lens with a focal length of 300 mm. The light were then separated by a polarization beam splitter (PBS) into horizontal and vertically components which were independently directed toward two line-scan CCD cameras of 2048 and a line scan acquisition rate of 70 kHz (AVIIVA M2 CL 2014, Atmel, CA, USA). Digitized spectral fringe profiles from the camera were acquired by data acquisition (DAQ) boards and transferred to a computer for further processing. DAQ boards and the galvanometer-based mirror were synchronized by a signal generated by an analog output device (PCI-6733, National Instruments). The data acquisition and transfer were triggered by a signal generated by a computer synchronized with the ramp function that drives the x-direction scanning galvanometer. The polarization controller (PC1) was aligned to modify the polarization of the light from the source to ensure maximum power. PC2 and PC4 allowed us to adjust the polarization of the light to achieve equal reference illumination power at the detector outputs in the SMF-based system.
Figure 1. Schematics of the polarization-sensitive spectral domain OCT system (a) and photo of the handheld sample arm (b). PC - polarization controller, P - polarizer, FC - fiber coupler, GM - galvanoscanner mirror, FL - focus lens, TG - transmission grating, M - mirror, CCD - line-scan CCD camera, SLD - superluminescent diode, PBS - polarizing beam splitter.
Download figure:
Standard image High-resolution image2.2. Muller matrix analysis of polarization properties of tissue
In the SMF-based system, the polarization information of sample and system are mixed together. It is difficult to directly extract the sample polarization information from the output signals with single IPS because the polarization information of fiber and fiber components are unknown. It has been shown that to extract the polarization properties of sample, it is required that the phase retardance of SMF system to be equal to a half-wave plate or full-wave plate by adjusting PCs.
In our analysis, the Stokes vector is used to represent the polarization state of the light from the horizontal and vertical (H & V) channels, and Muller matrix is used for describing the effects of SMF and optical components, and sample on the polarization of the light. Let H(z) and V(z) donate the interference fringe signals from a specific depth z, the output Stokes vector from spectrometer can be expressed as:
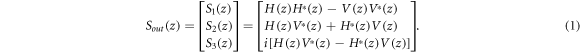
For a circularly polarized incident light and a bulk PS-OCT system, the Stokes vector of the backscattered light is [6, 34]:
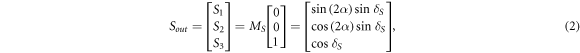
The phase retardance and optical axis orientation of sample are then given by:
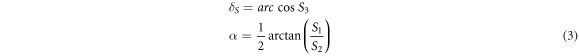
To derive the information about the changes in polarization state, we will use a modified model of the Muller matrix measurement. Figure 2 shows a simplified schematic of Muller matrix measurement with an all SMF-based PS-SDOCT, where Min represents Muller matrix of the illumination optics, Mout is the Muller matrix of the fiber path from the sample surface to the interferometer output, and Ms is a round-trip cumulative Muller matrix of a sample, Sout (zsurf) and Sout (z) are the Stokes vectors of the light from surface and the specific depth z of the sample, respectively. With the help of schematic in figure 2, equation (1) can be rewritten as:
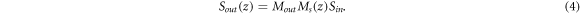
Figure 2. The schematic of the SMF-based PSOCT system with a single IPS.
Download figure:
Standard image High-resolution imageThe output Stokes vector from the sample surface in the system, Sout (zsurf) could be written as:
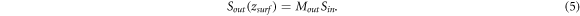
Note that we do not change the coordinate orientation for back-reflected light. Thus, the light reflected from the surface is equal to Sin. From equation (5), we have:
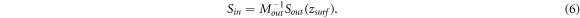
On substituting (6 ) into equation (4), we have:
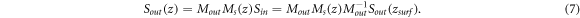
Equation (7) shows that Ms is mixed with Mout in the measured signals. For a linear retardar with an optical axis θ and retardance δ, its general expression can be written as:
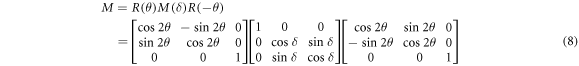
In our system, diattenuation of the polarizing components can be neglected. In this case, the unknown Muller matrix Mout, can be expressed as a product of a linear retardance matrix ML and circular retardance matrix MC:
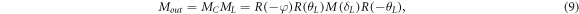
where φ is the circular phase retardance value of circular retardance matrix, and θL and δL are the optical axis orientation and the phase retardance of linear birefringence, respectively.
For an ideal sample with an optical axis orientation θS and the round-trip cumulative phase retardance δS, its general expression MS can be written as:
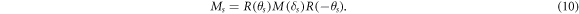
Note that Mout is an orthogonal matrix [18], we have  By substituting equations (9) and (10) into equation (7), we have:
By substituting equations (9) and (10) into equation (7), we have:
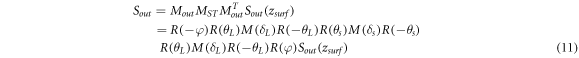
By adjusting the polarization controller in the sample arm (PC3), the system phase retardance is equal to that of a half-wave plate or full-wave plate, the output Stokes vector of the light refelceted from the sample surface is ![${\left[0,{\rm{0}},{\rm{1}}\right]}^{T}.$](https://content.cld.iop.org/journals/2399-6528/3/1/015014/revision2/jpcoaafefbieqn2.gif) Thus, M(δL) is expressed as:
Thus, M(δL) is expressed as:
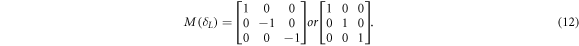
Therofore,  could be considered as a new retardar with an axis orientation α and phase retardance δs, equation (11) could be simplified as:
could be considered as a new retardar with an axis orientation α and phase retardance δs, equation (11) could be simplified as:
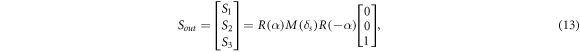
where 
If the SMF system is so stable enough that φ and θL remain unchanged, the phase retardance δS and axis orientation θs of the sample can be extracted from equation (3) and can be expressed as:
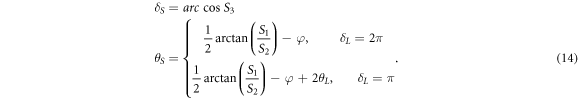
Equation (13) shows that when the output Stokes vectors from the sample surface is ![${\left[0,{\rm{0}},{\rm{1}}\right]}^{T}$](https://content.cld.iop.org/journals/2399-6528/3/1/015014/revision2/jpcoaafefbieqn5.gif) and the system retardance is equal to π or 2π by employing the PC3, the optical axis orientation and phase retardance of the sample can be measured with the SMF-based system. These prerequisites of system adjustment can be realized by using a quarter wave plate (QWP) [27]. In this case, the light incident on the sample is a circular polarized light, which can be verified by calculating the Stokes vector Sinof the light incident on the sample with equation (5).
and the system retardance is equal to π or 2π by employing the PC3, the optical axis orientation and phase retardance of the sample can be measured with the SMF-based system. These prerequisites of system adjustment can be realized by using a quarter wave plate (QWP) [27]. In this case, the light incident on the sample is a circular polarized light, which can be verified by calculating the Stokes vector Sinof the light incident on the sample with equation (5).
2.3. Removing multi-scattered light
In OCT imaging, the single scattered signal is particularly useful as it provides the localized optical information about the scatterers; while the multiply scattered signals photons degrades the detected signal. Therefore, determination of the tissue scattering coefficient, quantitative depth ranging, and flow measurement with OCT are generally based on single backscattered light. However, in tissue imaging, multiple scattered light is also present in the OCT signals due to the finite collection numerical aperture of the objective lens in the sample arm and partial coherence of the multiply scattered light.
In PS-SDOCT system, the light incident on the sample is illuminated by a polarized beam. The light measured by spectrometer has two components: single backscattered light Is and multi-scattered light Im. Is is decomposed into two orthogonal components  and
and  components in horizontal and vertical channels, respectively. If we use
components in horizontal and vertical channels, respectively. If we use  and
and  to represent multiply scattered light in the horizontal and vertical channels, we have
to represent multiply scattered light in the horizontal and vertical channels, we have  [33], due to the multiple scattering processes. Thus, the residual spectra of the emerging light can be expressed as:
[33], due to the multiple scattering processes. Thus, the residual spectra of the emerging light can be expressed as:
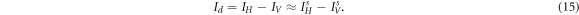
It can be seen from equation (15) that the contributions of the multiply scattered light are removed, resulting in a potential improvement of the signal-to-noise ratio (SNR) of the image.
3. Experimental results
3.1. Measurements of tissue samples
In order to prove the ability of our SMF-based PS-SDOCT system, different tissues, including in vivo finger nail and fingertip skin, ex vivo pork cartilage, and normal and burned chicken breast have been imaged. Figure 3 shows the intensity, phase retardation and optical axis orientation images of pork cartilage ex vivo, human finger nail, fingertip skin and normal and burned chicken breast, where glycerin was applied on the tissue surface before the imaging. The PS-SDOCT system was configured at an A-line acquisition rate of 70 kHz and consisted of 500 A-lines per image frame at a scanning width of 2 mm with the maximum imaging depth of 2.5 mm. Figure 3(a) was the intensity image which showed relatively homogeneous structure in the pork cartilage. The periodical stripe appearances in the phase retardation image figure 3(b), and optical axis orientation image figure 3(c) distributed in the PS-OCT signals were easily observed due to the existence of strong birefringence in pork cartilage. The retardance on the tissue surface was zero (black) and it changed with the increase of depth in the tissues. The PS images of in vivo finger nail were presented in figures 3(d)–(f). Figures 3(e) and (f) were phase retadance and optical axis orientation that also indicated distinct layered patterns. It was obvious that the phase retardation in figure 3(e) varied between 0 and 180 deg with the imaging depth. For the intensity image of fingertip skin in figure 3(g), it was easy to observe the typical layered structure of human skin, including the thick stratum corneum and dermis. For the polarization images in figures 3(h) and (i), it was evident that tissue birefringence varied within the images: the phase retardation in some regions was high (yellow) while the retardation in neighboring regions remains low (black). Consequently, these results show that our all SMF-based PS-SDOCT system with a single IPS is able to achieve quantitative measurements of phase retardance in biological tissues.
Figure 3. The intensity (a), phase retardation (black, 0°; yellow, 180°) (b) and optical axis orientation (black, 0°; yellow, 180°) (c) images of ex vivo pork cartilage. The intensity (d), phase retardation (black, 0°; yellow, 180°) (e) and optical axis orientation (black, 0°; yellow, 180°) (f) images of human finger nail. The intensity (g), phase retardation (black, 0°; yellow, 180°) (h) and optical axis orientation (black, 0°; yellow, 180°) (i) images of human fingertip skin. The intensity (j), phase retardation (black, 0°; yellow, 180°) (k) and optical axis orientation (black, 0°; yellow, 180°) (l) images of normal and burned chicken breast. Scale bar: 200 μm. The images are 2 mm wide × 1.5 mm in physical depth. sc - stratum corneum, d - dermis.
Download figure:
Standard image High-resolution imageFigures 3(j)–(l) present the cross sectional images of structural, phase retardation and optical axis orientation imaging of normal and burned chicken breast, respectively. The images were obtained by scanning the probing beam of light across the boundary of normal and burned breast of chicken in vitro. The right part of the images in figures 3(j)–(l) was the images of the burned structure. The left part of the images in figures 3(k) and (l) demonstrated the chnages in polarization properties of chicken breast before being heated. Note that in the normal chicken breast, the variation of the relative phase with depth could be seen clearly and the characteric structures disappeared in the heated part of the tissue. It was also noted that there existed a visible boundary between the normal and the burned area in the phase retardance image (see the blue arrow in figure 3(k)), which was not evident in the intensity image (see figure 3(j)).
3.2. Removing the effects of the multiply scattered light
To demonstrate the capability of our method for removing the effects of the multiply scattered light, figure 4 shows cross-sectional images in human fingertip skin. Figures 4(a) and (b) were obtained by the two CCDs, respectively, and figure 4(c) was achieved by spectral subtraction to eliminate the multi-scattering light in tissues from equation (15). On comparison of the images, it was easily observed that the results were quitely similar to those obtained in the previous experiment results, and figure 4(c) had the higher image contrast than the other images. Here, it is evident that multiple scattering light acts as background noise, seriously degrading image quality. Compared regions with the white arrows in figures 4(a)–(c), it was obvious that the interface of stratum corneum and dermis in figures 4(a) and (b) was blurred, and the interface in figure 4(c) was really clear and continuous. Thus, the noise artifacts were greatly suppressed by removing the multi-scattered light. In addition, from the red circles in three structural images of fingertip skin, depth range in figure 4(c) was also extended due to the increasing sensitivity.
Figure 4. The cross-sectional images (a), (b) and (c) of human fingertip skin. The images (a) and (b) obtained by two CCDs. The image (c) was achieved by spectral subtraction without multi-scattered light. Three curves CCD1, CCD2 and PRO were the mean intensity of A-lines between yellow dotted lines from (a), (b) and (c) in Plot (d). Scale bar: 200 μm. The images are 2 mm wide × 1.5 mm in physical depth. sc - stratum corneum, d - dermis.
Download figure:
Standard image High-resolution imageFigure 4(d) showed the mean intensity of A-scans in selected regions from figures 4(a)–(c) at several depths. It is obvious to directly observe the signal improvement without multi-scattered light, especially at depths from 450 μm to 700 μm.
To quantify the improvement of our method, the SNR of cross-sectional images was analyzed. First choosing regions in the structural image in human skin (marked as the blue rectangle and the green rectangle in figure 4(a)), the signal and noise regions are represented by the image gray scale. Here The SNR is defined as:
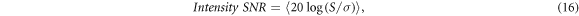
where the angle brackets represent the average over the values of selected regions. It should be noted that the intensity SNR evaluated here indicates a metric for image contrast, rather than the sensitivity of the system used. Similarly, the SNR of corresponding regions in figures 4(b) and (c) could be calculated. The SNR values of two images in figures 4(a) and (b) obtained by two CCDs were 48.9 dB and 49.5 dB. In contrast, by spectral subtraction to eliminate the multi-scattering light from equation (16), the SNR of the image in figure 4(c) was increased to 52.1 dB. As a consequence, it was obvious that our method resulted in a SNR increase of at least 2.6 dB comparing to single CCD.
4. Discussion and conclusions
In conclusion, we have developed a PS-SDOCT system with a single IPS based on all SMF. We used two high-speed line-scan CCD cameras and a PBS to detect the two orthogonal polarization components by using a single spectrometer. Stokes vector is used to represent the polarization state of the light from the horizontal and vertical channels, and Muller matrix is used for describing the effects of SMF and components, and sample in the system. Based on Mueller matrix analysis, the fiber system and sample could be transferred to a new retardar by employing PCs, by which the phase retardance and optical axis orientation of sample could be extracted. In this case, the effects of fiber birefringence of SMFs and SMF components were corrected. Output Stokes vector as circular polarized light from the sample surface and equal reference illumination power at the detector outputs were provided by PCs, which were key elements to the proper functionality of the system. Images of the intensity, phase retardation and optical axis orientation images in pork cartilage, finger nail and fingertip skin were obtained to demonstrate the capability of our method. In addition, all SMF-based PS-SDOCT system demonstrated the improvement of image quality by reducing multi-scattering component from the biomedical tissues. The SNR in the cross-sectional image of human fingertip skin was increased by at least 2.6 dB.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 61275198, 60978069).





