Abstract
This paper describes a high-throughput method for developing physically modified chitosan membranes to probe the cellular behavior of MDCK epithelial cells and HIG-82 fibroblasts adhered onto these modified membranes. To prepare chitosan membranes with micro/nanoscaled features, we have demonstrated an easy-to-handle, facile approach that could be easily integrated with IC-based manufacturing processes with mass production potential. These physically modified chitosan membranes were observed by scanning electron microscopy to gain a better understanding of chitosan membrane surface morphology. After MDCK cells and HIG-82 fibroblasts were cultured on these modified chitosan membranes for various culture durations (i.e. 1, 2, 4, 12 and 24 h), they were investigated to decipher cellular behavior. We found that both cells preferred to adhere onto a flat surface rather than on a nanopatterned surface. However, most (> 80%) of the MDCK cells showed rounded morphology and would suspend in the cultured medium instead of adhering onto the planar surface of negatively nanopatterned chitosan membranes. This means different cell types (e.g. fibroblasts versus epithelia) showed distinct capabilities/preferences of adherence for materials of varying surface roughness. We also showed that chitosan membranes could be re-used at least nine times without significant contamination and would provide us consistency for probing cell–material interactions by permitting reuse of the same substrate. We believe these results would provide us better insight into cellular behavior, specifically, microscopic properties and characteristics of cells grown under unique, nanopatterned cell-interface conditions.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Corrections were made to this article on 19 August 2013. The name of the final author was amended.
1. Introduction
This paper describes an easy-to-handle, facile approach—a combination of multiple IC-based manufacturing processes—to prepare nanopatterned chitosan membrane substrates (with two alternatives) to probe the cellular behavior of two types of mammalian cells—Madin-Darby canine kidney (MDCK) epithelial cells and HIG-82 rabbit synobyl fibroblasts. Individual cells were exposed to various physical stimuli, such as fluidic shear stress or substrate rigidity [1–3]. Subsequent intracellular signals, in turn, modulate the dynamic responses of intracellular proteins, causing observable, measurable cellular behavior noted herein. Cells are also influenced by numerous non-specific external stimuli (i.e. non-physically based stimuli) such as changes in either pH value or temperature, electrical fields and the surface chemistry of the adhesive substrate [1]. To fully understand how single mammalian cells sense and then respond to these stimuli, whether multiple or singular in nature, has been a longstanding but interesting subject in multiple research communities such as biomaterials, cell biology, tissue engineering and mechanobiology [4, 5].
Substrate properties influence cellular behavior including a cell's protein reconstruction; this offers opportunities for specific ligand–receptor interactions (e.g. actin filament remodeling or integrin redistribution) [1, 6] and options for leveraging general surface chemistry properties such as relative surface charge and hydrophilicity [7, 8]. Cell–material interfaces and corresponding responses can alter the physiological functionality of single mammalian cells, significantly affecting the success of implantable medical devices. For example, modified silicone microtopography (10 μm vertical projections) has been shown to inhibit the over-proliferation of rat neonatal cardiac fibroblasts when co-culturing cardiac myocytes with rat neonatal cardiac fibroblasts [9]. In this study by Boateng et al [9], excessive fibroblast proliferation in cardiac myocyte cultures was decreased by 50% after 5 days of culture, indicating that microtopographic properties (i.e. variations from planar to raised/uneven) can effectively prevent non-myocyte proliferation. Numerous micro- and nano-engineering approaches have been used to control cell–substrate interactions in vitro by presenting specific molecules to cells [10–16]. For example, one of the first applications using a microengineering approach to control cell microenvironment was demonstrated by Whitesides et al [10–13]. In their studies, they used soft lithography to fabricate microstructures to generate micropatterned surfaces comprised of adhesive regions to which cells could attach. In addition, self-assembled monolayers or self-assembled multi-layer structures have been shown to affect the behavior of neurons [14], myoblasts [15] and fibroblasts [16]. The surface chemistry and topography of the substrate may be important in directing cellular behavior such as proliferation, migration and matrix deposition. Flemming et al [17], provided an extensive review of the effects of surface topography on cellular behavior. In the last decade, the ability to fabricate and evaluate the effect of nanosized topographical features similar to the extracellular matrix (ECM) has allowed researchers to more accurately represent and examine cells in a physiological environment.
Most previous in vitro studies have examined cells cultured on a two-dimensional flat and rigid substrates; in vivo, however, most cells are exposed to a three-dimensional microenvironment with complex topographical features. Using some advanced nanofabrication methods, nanoscale topographical features can be incorporated into the in vitro experimental platform to mechanically and structurally mimic various in vivo three-dimensional ECM environments. Qi et al studied the interactions between biological cells and vertically aligned silicon nanowire (SiNW) arrays (fabricated by self-assembling nanoelectrochemistry). They focused on understanding how SiNW arrays affect cellular behavior such as cell adhesion and spreading [18]. The results of this study show that SiNW arrays can not only enhance the cell–substrate adhesion force but also restrict cell spreading. Pesen et al [19] used electron beam lithography to pattern nanostructures and two different combinations of applied proteins to examine cellular mechanisms associated with the formation and organization of focal adhesions. It was found that the very shape of nanoscale surface patterns can be used to modulate focal adhesion complex organization and determine the organization of the adjoining cytoskeleton. Haq et al [20] used nanopillars and nanopores with dimensions comparable too, but slightly larger than, those of filopodia (∼ 100–150 nm) to investigate the electrophysiological activities of neurons. They found that PC 12 cells exhibited a higher density and shorter neurite extension on nanopillars, but a lower density and intermediate neurite extension when grown on nanopores. Nanopillars had a greater inhibiting effect on neurite outgrowth than nanopores. Cecchini et al [21] used nanogratings to analyze the differentiation of PC 12 cells. He concluded that topographic cues were more effective in determining axon outgrowth direction than cell–cell interactions. His results showed unstable synaptic connections and cell-body polarization, and further suggested that the substrate topography can act cooperatively with nerve growth factor signaling to regulate cell differentiation and neurite outgrowth. These approaches have allowed researchers to probe biologically relevant issues using advanced nanotechnologies. However, the main drawbacks of these approaches include the following: (i) the challenges and time required to fabricate nanotopography (e.g. it is time consuming to fabricate nanostructures by using either electron beam or electrospinning); and (ii) difficulties with mass production for further applications (e.g. tissue engineering).
The challenges in tissue engineering include the following: (i) the generation of vascularized tissues and complex geometries is difficult due to our limited ability to control the cellular environment at the micro/nanoscale; (ii) the lack of suitable materials with the desired degradation rates and the mechanical properties for the desired tissue; (iii) the optimization of scaffold architecture, including pore size, bioactivity, surface topography and morphology. Therefore, new and optimized processing methods must be developed to address issues related to cell seeding, vascularization and scale up into three-dimensional structures. Chitosan-based materials (or chitin-based materials) originate from nature, they have been approved by the US Food and Drug Administration (FDA) and Environmental Protection Agency (EPA) and are used in a variety of biotechnologically relevant applications, such as drug carriers and nanoparticles [22, 23]. However, it is still challenging to produce surface modifications, such as nanostructures, on chitosan membranes. Herein, extending our previous work [1–3, 8, 24], we have developed a robust and easy to build and operate method to fabricate nanoscale topographical features and mesa structures (positive and negative forms) on chitosan surfaces to study behavior of MDCK cells and HIG-82 fibroblasts. In this study, the physical nanofabrication approach was developed through the following steps: (i) mesa structures were defined on monocrystalline silicon wafers using a UV-photolithography process followed by treatment with Ag-nanoparticles (AgNPs assisted etching) to form nanopatterns [8]; (ii) the positively and negatively nanopatterned chitosan membranes were prepared through a combination of solution casting, drying and peeling off from silicon nanopatterns; and, (iii) the mammalian cells—MDCK cells and HIG-82 fibroblasts—were cultured on nanopatterned chitosan membranes to probe cellular behavior.
2. Materials and methods
2.1. Preparation of positively and negatively nanopatterned silicon molds
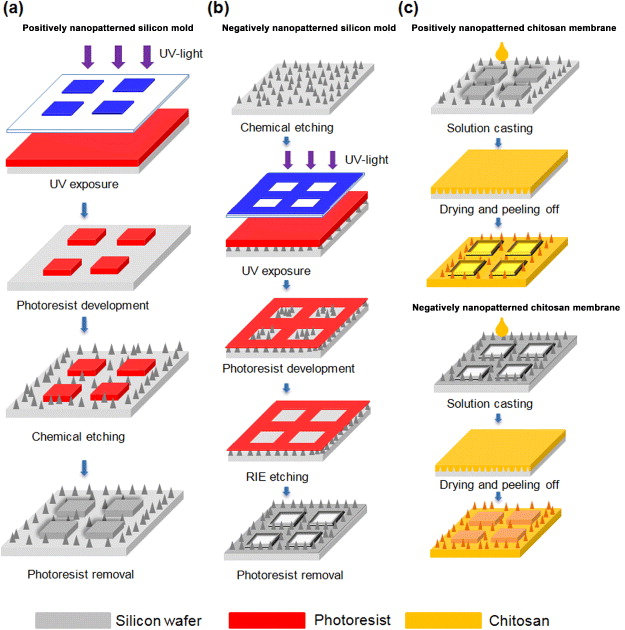
First, the positive silicon mold was made by photolithography to create desired patterns on a 4-inch monocrystalline silicon wafer. The patterned silicon wafer with photoresist was then dipped into a 0.01 M silver nitrate solution for 5 min. The number of silver ions (Ag+) decreased on the Si surface because of the higher electronegativity of Ag than Si. After formation of a metallic catalyst layer, the wafer was immersed in an etchant comprising hydrogen fluoride (HF, 49 wt%) and hydrogen peroxide (H2O2, 30 wt%), with a mixture ratio of 3:1 (v/v), for 1.5 min at room temperature. After etching, the positively nanopatterned silicon mold was prepared by photoresist removal with acetone and rinsed with de-ionized water (figure 1(a)).
Figure 1. (a) Fabrication of positively nanopatterned silicon molds. The designed mesa structures and silicon nanopatterns were fabricated through a combination of photolithography and chemical etching on silicon wafers. The positively nanopatterned silicon mold was created, after photoresist removal by acetone, and it was cleaned with deionized water. (b) Fabrication of negatively nanopatterned silicon molds. First, the silicon nanopatterns were fabricated through chemical etching on silicon wafers. The designed planar patterns were then fabricated by a photolithography process, and the silicon nanopatterns were removed by reactive ion etching for 6 min, followed by oxygen plasma treatment for 8 min to remove photoresist. (c) Chitosan solution with suitable viscosity to flow into the silicon nanopatterns was then placed onto positively and negatively nanopatterned silicon molds. After solvent evaporation, the chitosan molecule formed a membrane with a nanopatterned surface that replicated the morphology of silicon nanopatterns. The nanopatterned chitosan membranes were peeled off after the removal of silicon nanopatterns using 0.1 M NaOH.
Download figure:
Standard image High-resolution imageThe negative silicon mold was made by dipping a 4-inch monocrystalline silicon wafer into a 0.01 M silver nitrate solution for 5 min and then immersing in an etchant comprising HF (49 wt%) and H2O2 (30 wt%), with a mixture ratio of 3:1 (v/v), for 1.5 min at room temperature. The desired patterns were completed using a photolithography process after coating photoresist on nanopatterned silicon wafer. The negatively nanopatterned silicon mold was prepared by reactive ion etching (P300, Integrated Plasma Incorporated Limited Co., Taiwan) for 6 min and photoresist removal by an oxygen plasma stripper system (P300, Integrated Plasma Incorporated Limited Co., Taiwan) for 8 min (figure 1(b)).
2.2. Preparation of positively and negatively nanopatterned chitosan membranes
The 1% (w/v) chitosan solution was prepared by dissolving medium molecular weight chitosan powder (190–310 kDa; Sigma-Aldrich; St Louis, MO, USA) in 1% (v/v) acetic acid solution. The solution was continuously stirred with a sterile magnetic bar for 6 h, and undissolved impurities and foam were removed by vacuum filtration and a Whatman no. 541 filter paper (0.22 μm pore size). After casting chitosan solution on positively and negatively nanopatterned silicon molds, the nanopatterned chitosan membranes were carried out by drying in an oven at 60 °C for 6 h and peeling off from silicon molds by immersing into 0.1 M sodium hydroxyl solution for 4 h (figure 1(c)). The nanopatterned chitosan membranes were finally rinsed with de-ionized water until neutral pH value was achieved.
2.3. Cell culture
Rabbit synobyl fibroblasts (HIG-82) and MDCK cells were maintained in normal tissue culture T-25 flasks with Ham's F-12 medium (Invitrogen, Carlsbad, CA, USA) for HIG-82 fibroblasts and Dulbecco's Modified Eagle's Medium (Invitrogen, Carlsbad, CA, USA) for MDCK epithelial cells. Both media were supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories Inc., Rockford, IL, USA), 4 mM l-glutamine, 100 U mL−1 penicillin and 1.5 g L−1 sodium bicarbonate, and culture were maintained at 37 °C in a humidified 5% CO2 incubator. Cells were trypsinized with 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) and re-seeded onto the sterilized nanopatterned chitosan membranes (positive and negative membranes) at a density of 5 × 104 cells mL−1 for 12 h to perform experiments and examinations.
2.4. Scanning electron microscopy (SEM)
The morphology of silicon molds and chitosan membranes was examined with a high-resolution field-emission SEM—Ultra 55 (Carl Zeiss SMT AG, Germany). The chitosan membranes were dried in air and then sputtered with gold at a current of 15 mA for 3 min by using an ion sputterer (E-1010; Hitachi, Japan). The MDCK cells and HIG-82 fibroblasts adhered onto positively nanopatterned chitosan membranes visualized with SEM were first fixed with a fixative containing 2.5% glutaraldehyde per 0.1 M PBS at 4 °C for 30 min. After washing twice with 0.1 M PBS, the cells were post-fixed with 1% osmium tetroxide (Sigma-Aldrich, St Louis, MO, USA) at room temperature for 30 min. The cells were then washed twice again with 0.1 M PBS, dehydrated through serial gradients of ethanol (10 min for each gradient, 50, 70, 80, 90 and 100), and finally dried out in a critical point dryer (HCP-2; Hitachi, Japan). The cells on positively nanopatterned chitosan membranes were sputtered with gold at a current of 15 mA for 3 min by an ion sputterer.
2.5. Epi-fluorescence microscopy
MDCK cells and HIG-82 fibroblasts were cultured on positively nanopatterned chitosan membranes and then washed twice with PBS. The cells were fixed with fresh 4% paraformaldehyde for 15 min at room temperature. After washing with PBS two more times, the substrates were immersed in 0.1% Triton X-100 for 10 min to permeabilize cell membranes. Rhodamine-conjugated phalloidin (Invitrogen, Carlsbad, CA, USA) was used to label actin filaments in living cells, while 4',6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA, USA) was used to stain DNA in nuclei. The cells were mounted with Fluoromount-G (Southernbiotech, Birmingham, AL, USA), and sealed under a coverslip. The cells were examined under an epi-fluorescent microscope (Zeiss Axio Observer, Germany) equipped with a 10 × eyepiece and 63 × (NA 1.4) objective to image their morphology and actin filaments.
2.6. Atomic force microscopy
An atomic force microscope (Bioscope AFM, Digital Instruments, USA) operated in the tapping mode was employed in this study to examine the topography of chitosan membranes. A rectangular NCH cantilever (Nanoworld AG, Switzerland) with a nominal spring coefficient of 42 N m−1 and a resonance frequency of 320 kHz was used. The cantilevers were cleaned with ethanol and immediately rinsed with de-ionized water before use. Topographic images were captured and analyzed using commercial software (Nanoscope (R) III v5.31R1).
3. Results and discussion
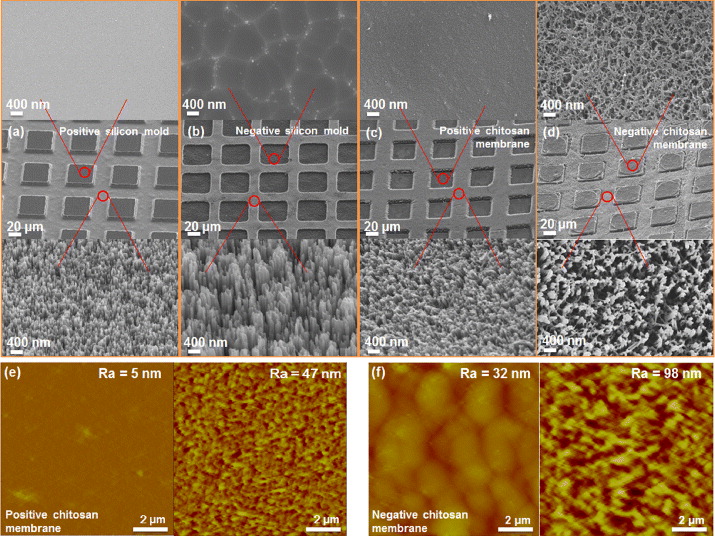
Figure 2 shows SEM images of positively and negatively nanopatterned silicon molds and nanopatterned chitosan membranes. The morphology of mesa structures indicated a planar surface surrounded by nanopatterns. Note, we also designed variously sized mesa structures (i.e. 60, 80, 100 μm). The height between mesa structures and nanopatterns on both positively and negatively nanopatterned silicon molds was about 4 μm, and the diameter of each nanopattern was 80–100 nm (figures 2(a) and (b)). The mesa structures on negatively nanopatterned silicon molds were damaged and showed some artifacts compared to the positively nanopatterned silicon molds due to reactive ion etching process and photoresist removal by oxygen plasma (figures 2(a) and (b)). After the remolding process, both the positively and negatively nanopatterned chitosan membranes could be fully transferred from the silicon molds. However, the surface of mesa structures on negatively nanopatterned chitosan membranes was not that smooth because the chitosan solution replicated the residual photoresist on the silicon mold after oxygen plasma cleaning (figures 2(c) and (d)). The topology and surface roughness of mesa structures and nanopatterned surfaces of positive and negative chitosan membranes were also examined by atomic force microscopy, respectively (figures 2(e) and (f)). The surface roughness of mesa structures and nanopatterns on positively nanopatterned chitosan membranes were 5 ± 1 nm (n = 5, N = 3) and 47 ± 8 nm (n = 5, N = 3), respectively. The surface roughness of mesa structures and nanopatterns on negatively nanopatterned chitosan membranes was 32 ± 4 nm (n = 5, N = 3) and 98 ± 7 nm (n = 5, N = 3), respectively. Further experiments were conducted after chitosan membranes were sterilized by UV-light for 2 h.
Figure 2. Scanning electron microscopy morphologies of mesa structures and nanopatterns on (a) a positively nanopatterned silicon mold, and (b) a negatively nanopatterned silicon mold. The mesa structures on negatively nanopatterned silicon molds were damaged and showed some artifacts compared to positively nanopatterned silicon molds due to photoresist removal by oxygen plasma. Morphologies of mesa structures and nanopatterns of (c) positively nanopatterned chitosan membranes, and (d) negatively nanopatterned chitosan membranes. The surface of mesa structures on negatively nanopatterned chitosan membranes was not smooth because the chitosan solution replicated the residual photoresist on negatively nanopatterned silicon molds after oxygen plasma cleaning. Surface topologies of mesa structures and nanopatterns of (e) positively nanopatterned chitosan membranes, and (f) negatively nanopatterned chitosan membranes.
Download figure:
Standard image High-resolution imageTwo types of mammalian cells—MDCK (epithelial) and HIG-82 (fibroblast)—were cultured on both positively and negatively nanopatterned chitosan membranes for various culture durations (i.e. 1, 2, 4, 12 and 24 h) to study cellular behavior on the membranes. During culture times, these cells were observed with an optical microscope to study cellular behavior on positively and negatively nanopatterned chitosan membranes. Figures 3(a) and (b) show phase contrast images of HIG-82 fibroblasts after culturing for various culture durations on positively and negatively nanopatterned chitosan membranes, respectively. These images indicate that HIG-82 fibroblasts cultured on positively and negatively nanopatterned chitosan membranes adhere onto the constructed planar surfaces. Relatively few of the HIG-82 fibroblasts spread out on the constructed planar surface of the positively and negatively nanopatterned chitosan membranes (compared to MDCK cells) before 12 h culture, because HIG-82 fibroblasts need a longer culture time to proliferate and spread (i.e. 24 h culture). Figures 3(c) and (d) show phase contrast images of MDCK cells after culturing for various culture durations on positively and negatively nanopatterned chitosan membranes, respectively. The images show that MDCK cells cultured on positively nanopatterned chitosan membranes adhere and spread out at the constructed planar surface within 4 h. However, most (> 80%) of the MDCK cells showed rounded morphology and would suspend in the culture medium instead of adhering onto the planar surface of negatively nanopatterned chitosan membranes. Figure 3(e) shows the percentage of HIG-82 fibroblasts and MDCK cells adhered onto flat surfaces with various sizes of the positively and negatively nanopatterned chitosan membranes at various culture durations. Results indicate that, for HIG-82 fibroblasts cultured on both positively and negatively nanopatterned chitosan membranes, cells preferred to adhere onto the flat surfaces rather than onto nanopatterned surfaces (> 90%); however, MDCK cells preferred to adhere onto the flat surface of positively nanopatterned chitosan membranes. Surface roughness (or topography) is one of the important factors influencing cell adhesion and proliferation. Indeed, roughness has been shown to modulate the biological response of tissues in contact with implants [25–27]. The surface roughness has a direct influence in vitro as well as in vivo on cellular morphology, proliferation and phenotype expression. Depending on the scale of irregularities of the material surface, surface roughness can be divided into macro-roughness (100 μm–millimeters), micro-roughness (100 nm–100 μm) and nano-roughness (less than 100 nm), each with its specific influence [27]. Macro-roughness is favorable for cells, because it is larger than the cell size and is not usually felt by cells. Micro-roughness is more controversial, because different cell types (e.g. epithelia versus fibroblasts) respond differently depending on the scale of surface roughness (or topography) [28–31]. Nano-roughness has been found to have significant effects on cell response, such as cell adhesion and proliferation [8, 18, 19]. The topography of the mesa structures on negatively nanopatterned chitosan membranes (figure 2(d), surface roughness ∼32 nm (< 40 nm)) could be suitable for HIG-82 fibroblasts to adhere [32], but not MDCK cells. The topography of nanopatterns on both nanopatterned chitosan membranes (figures 2(c) and (d), surface roughness ∼50–100 nm (> 50 nm)) could be unsuitable for MDCK cells and HIG-82 fibroblasts to attach and restrict cell spreading.
Figure 3. Phase contrast images of HIG-82 fibroblasts after 1, 2, 4, 12 and 24 h cultures on (a) positively nanopatterned chitosan membranes, and (b) negatively nanopatterned chitosan membranes. Phase contrast images of MDCK cells after 1, 2, 4, 12 and 24 h cultures on (c) positively nanopatterned chitosan membranes, and (d) negatively nanopatterned chitosan membranes. Both cells preferred to adhere onto a flat/planar surface rather than on nanopatterns of positively nanopatterned chitosan membranes. HIG-82 fibroblasts could adhere onto rough surfaces better than MDCK cells could, based on the morphology of flat surfaces on negative nanopatterned chitosan membranes. (e) The ratio of MDCK cells and HIG-82 fibroblasts adhered onto a flat surface versus total cells with various widths of positively and negatively nanopatterned chitosan membranes. Data are mean ± standard deviation (N =5, n =10).
Download figure:
Standard image High-resolution imageFigures 4(a) and (b) show SEM images of MDCK cells and HIG-82 fibroblasts cultured on positively nanopatterned chitosan membranes for 12 h. The MDCK cells spread after 12 h culture; however, HIG-82 fibroblasts showed a rounded shape with less spreading. We also examined cell morphology using actin filament staining as shown in figures 4(c) and (d). Figures 4(c) and (d) show phase contrast images combined with fluorescence images of MDCK cells and HIG-82 fibroblasts with labeled cytoskeleton and nuclei cultured on positively nanopatterned chitosan membranes for 12 h, respectively. After culturing cells on positively nanopatterned chitosan membranes of suitable size or geometry, we can investigate the interaction between single cells and surrounding microenvironments (figure 4(d)). We also examined the reusable capacity of our manufactured chitosan membranes through the following steps: (i) MDCK cells were cultured on positively nanopatterned chitosan membrane for 24 h to form the desired patterns; (ii) the chitosan membrane was rinsed twice with PBS after MDCK cells were detached by trypsin-EDTA; and, (iii) MDCK cells were re-seeded on the chitosan membrane for another 24 h of culture. We found that our chitosan membranes could be re-used at least nine times (figure 4(e)). In addition, we used Annexin-V conjugated fluorescein isothiocyanate (FITC) to label the apoptotic cells to examine whether these re-used chitosan membranes were contaminated. In the ninth cycle, approximately 3% ± 1% (n = 10, N = 3) of MDCK cells underwent apoptosis, implying that no significant defects or contamination were found on these membranes. This application can provide us the consistency for probing cell–material interactions by reusing the same substrate. For example, the ideal stem cell culture platform would support long-term expansion (> 20 passages) of undifferentiated stem cells, maintain efficacy in defined media, has compatibility with common sterilization techniques, results from a process that is scalable, reusable and relatively inexpensive, and demonstrates efficacy for multiple stem cell lines and types. Furthermore, we can also examine various cellular behavior at specific locations on the same substrate by detaching and reseeding different cells on these reusable chitosan membranes.
Figure 4. Scanning electron microscopy images of (a) MDCK cells, and (b) HIG-82 fibroblasts after 12 h of culture on positively nanopatterned chitosan membranes. The MDCK cells showed spread morphology, however, HIG-82 fibroblasts showed rounded shape with less spreading. Phase contrast images combined with fluorescence images of actin filaments (red) and nuclei (blue) through rhodamine-conjugated phalloidin and DAPI of (c) MDCK cells and (d) HIG-82 fibroblasts. (e) Schematic and phase contrast images of MDCK cells cultured on reusable nanopatterned chitosan membranes nine times. At the ninth cycle, apoptotic cells were labeled with Annexin V-FITC (green) and all cells were stained for nuclei (blue). In the ninth cycle, only around 3% ± 1% (N = 3, n = 10) MDCK cells underwent apoptosis which implied that no significant defects or contamination were found on these membranes.
Download figure:
Standard image High-resolution image4. Conclusions
In this experiment, we developed an approach to create nanopatterns on chitosan membranes, one of the US FDA/US EPA-approved biomaterials. These newly developed nanopatterned chitosan membranes would provide us a stable in vitro cell culture platform in order to obtain more comprehensive insight into cellular behavior and structural responses. In this study, we have summarized the following: (i) both HIG-82 fibroblasts and MDCK cells cultured on positively nanopatterned chitosan membranes would attach onto mesa structures, indicating that both cells preferred to adhere onto a flat surface rather than a nanopatterned surface; (ii) different cell types (e.g. fibroblasts versus epithelia) showed distinct capabilities/preferences for adherence onto materials of varying surface roughness; and, (iii) the constructed chitosan membranes could be re-used at least nine times without contamination. These promising results, we believe, would have a wide range of potential applications such as the development of naturally derived biomaterials, the design and manufacture of biomedical devices, in vitro diagnostic devices or platforms, and, ultimately, tissue engineering.
Acknowledgments
We thank the National Science Council of Taiwan for financially supporting this research under contract no. NSC 101-2623-E-007-005-ET (to JAY), NSC 101-2628-E-007-011-MY3 (to C-MC), and the Grant for Interactive Nano/MicroElectroMechanical Components and Systems from National Tsing Hua University, Taiwan (to JAY and C-MC).