Abstract
Carbon (C) release from thawing permafrost is potentially the largest climate feedback from terrestrial ecosystems. However, the magnitude of this feedback remains highly uncertain, partly due to the limited understanding of how abrupt permafrost thaw (e.g. permafrost collapse) alters soil organic matter (SOM) quality. Here we employed elemental analysis, stable isotope analysis, biomarker and nuclear magnetic resonance techniques to explore changes in soil C concentration and stock as well as SOM quality following permafrost collapse on the Tibetan Plateau. Our results showed that permafrost collapse resulted in a 21% decrease in soil C concentration and a 32% reduction in C stock of the top 15 cm of soil over 16 years. Moreover, permafrost collapse led to a significant decline in SOM quality: the relative abundance of labile SOM fractions (e.g. carbohydrates) decreased, whereas recalcitrant SOM fractions (e.g. suberin-derived compounds) increased 16 years after collapse. By contrast, the relative abundances of labile and recalcitrant compounds showed no significant differences in the control plots along the thaw sequence. These results demonstrate that permafrost collapse and consequent changes in soil environmental conditions could trigger substantial C release on decadal timescales, implying that abrupt thaw may be a dominant mechanism exposing soil C to mineralization.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Soils in the permafrost zone contain approximately one half of global soil organic carbon (C; ∼1300 Pg C) because of persistent cold and wet conditions (Tarnocai et al 2009, Hugelius et al 2014). Given that the permafrost regions are warming approximately twice as fast as the global average (Screen and Simmonds 2010), C release from thawing permafrost may be the largest positive climate feedback from terrestrial ecosystems (Grosse et al 2011, Koven et al 2011, Schuur et al 2015). Abrupt permafrost thaw (ground surface subsidence caused by thaw of ice-rich permafrost) could lead to the occurrence of thermokarst and thermo-erosion processes, which would dramatically alter the local landscape, affecting vegetation, soil and hydrology (Jensen et al 2014). This contrasts with top-down thawing that only impacts the permafrost surface (Schuur et al 2015). Especially on sloping landscapes such as uplands, this abrupt thaw process can greatly destabilize the ground surface and expose soil C to decomposition from metres below the surface (Pizano et al 2014, Abbott and Jones 2015). Despite increasing evidence that abrupt thaw accelerates soil organic matter (SOM) decomposition (Schuur et al 2009, Lee et al 2011), the magnitude and persistence of these effects are highly uncertain (Abbott and Jones 2015, Schuur et al 2015). Much of this uncertainty arises from the fact that SOM decomposition after abrupt thaw depends on environmental conditions (e.g. temperature and wetness) as well as the quality of SOM (Hodgkins et al 2014, Treat et al 2014, Chen et al 2016). The occurrence of abrupt thaw on sloping landscapes would increase soil temperature due to the high thermal conductivity of bare soils which have low albedo, and may also increase or decrease the soil moisture depending on microtopography (Jensen et al 2014, Abbott and Jones 2015). Therefore, better quantifying how changes in physical conditions alter SOM quality is crucial to predicting the timing and magnitude of feedbacks between the C cycle and climate change (Schädel et al 2014, Koven et al 2015).
Abrupt thaw can alter SOM quality via enhanced SOM decomposition, lateral C export, and accelerated plant growth and associated C inputs (Hodgkins et al 2014, Pizano et al 2014, Pearce et al 2015). In lowland environments, the development of bogs and fens after abrupt thaw-induced inundation and consequent vegetation succession, and thus altered C inputs and associated organic matter lability, finally leading to an increase in SOM quality (Hodgkins et al 2014). By contrast, abrupt thaw in upland landscapes (e.g. retrogressive thaw slumps and thermo-erosion gully features) generally decreases soil moisture and increases soil temperature, which appears to more strongly stimulate C loss via microbial decomposition than vegetation C inputs (Lawrence et al 2015, Schuur et al 2015). The contrasting dynamics of environmental conditions induced by abrupt thaw may exert distinct effects on SOM quality. However, current studies mainly focus on net ecosystem flux (Schuur et al 2009, Lee et al 2011, Abbott and Jones 2015), with little known about changes in SOM quality after the initiation of abrupt thaw. Particularly, how SOM quality varies with time after abrupt thaw remains uncertain, which is a primary source of uncertainty in predicting soil C dynamics (Lawrence et al 2015).
The Tibetan Plateau is the largest alpine permafrost region in the world (Zhang et al 2008), storing large quantities of soil C (Ding et al 2016). In contrast to the predominantly flat landscape of the Arctic tundra, the Tibetan Plateau has complex topography with abundant mountains (Royden et al 2008). This rugged terrain provides natural contrasts to explore the impacts of permafrost collapse on SOM quality. In this study, we explored a typical abrupt thaw feature in the upland region of the northeastern Tibetan Plateau to examine how permafrost collapse accompanied by soil environmental changes alters SOM quality. We used elemental analysis, stable isotope analysis, 13C nuclear magnetic resonance (NMR) spectroscopy, and biomarker techniques to quantify soil C concentration, stock as well as SOM quality. Based on these measurements, we tested the hypothesis that abrupt thaw-induced environmental changes (warming and drying) in upland regions would intensify SOM decomposition, potentially decreasing SOM quality.
2. Methods
2.1. Site description
The study site is located in Gangca county, on the northeastern Tibetan Plateau, China (37°28' N, 100°17' E, altitude of 3847 m). The hillslope where the abrupt thaw feature formed is a moist meadow landscape underlain by discontinuous permafrost (figures 1 and S1 is available online at stacks.iop.org/ERL/13/104017/mmedia). Mean annual temperature is −3.3 °C, and mean annual precipitation is 460 mm (Chen et al 2018). The vegetation consists of alpine swamp meadow, dominated by Kobresia tibetica, K. royleana and Carex atrofusca (Yang et al 2018a). The soil in this area has a silty loam texture with pH of 5.8. Based on GSSI georadar (SIR-20, Laurel, Santa Clara, USA) and thaw-probe measurements, the average active layer thickness (ALT) of this study area is 0.86 m (Yang et al 2018b). The ALT within and outside the thaw feature is 1.05 and 0.83 m, respectively (figure S2).
Figure 1. Study site and features of abrupt permafrost thaw on the Tibetan Plateau. Sampling locations were classified as early stage (1 year since collapse), middle stage (10 years since collapse), and late stage (16 years since collapse) of permafrost collapse.
Download figure:
Standard image High-resolution image2.2. Experimental design and soil sampling
In July of 2014, we sampled six transects (transect 1 to transect 6, hereafter referred to as TS1–TS6) along the thaw feature (figure S3). Transects were placed every 20 m perpendicular to the downslope axis of the feature resulting in a thaw sequence since permafrost collapse. Transects covered the whole width of the thaw feature and included paired sites in adjacent, undisturbed grassland as a control for possible non-permafrost-collapse changes associated with slope position. In each transect, we set up a collapse plot (∼20 m × 10 m) inside the feature and a paired control plot in the undisturbed grassland, respectively. To characterize microtopography, we generated a high-resolution topographic model of the landscape with LiDAR (VZ-400, Riegl, Horn, Austria, analyzed with Riscan pro 2.0 software) to measure the surface area of disrupted vegetated patches and exposed mineral soil within the feature (figures S1(b)–(e)). To determine time since collapse for the six transects, we estimated the rate of landscape retreat with field measurements from 2014 to 2016, and satellite images from Google Earth from 2007 to 2013 (figure S1(a)). The time since permafrost collapse along the thaw sequence was calculated as follows (equation (1)):

where Tcollapse is the collapse time of the plot within the feature (yr), Lretreat is the distance from the plot to the location of collapse initiation (m), and Vretreat is the estimated landscape retreat rate (m yr−1).
For each transect, we sampled the top 15 cm of soil with a 3 cm diameter hand corer. Within the thaw feature, soil samples were collected from the center of each disrupted vegetated patch at least 10 cm away from the edge of the patch. Patches were numbered and locations were noted by measuring the distance from each patch to two reference corners of the plot (figure S3). In the control plot, we sampled five quadrats at the corners and center of the plot following the same protocol as that for the impacted sites. Considering the experimental cost, the C and nitrogen (N) concentrations and bulk density were measured for all samples, but other soil parameters, such as soil moisture, pH, δ13C and SOM composition, were analyzed for only three transects with composite samples (TS1, TS4, and TS6). Specifically, both collapse and control plots within these three transects were divided into nine equal grid cells, respectively. All the soil samples were then assigned to one of these nine grid cells according to their location. Soil samples within the three diagonal grid cells were selected and mixed separately to obtain three composite samples, which were used for subsequent analyses (figure S3; n = 3).
2.3. Soil physical and chemical analysis
We determined soil C and N concentrations as well as bulk density for samples collected from all transects. Soil C and N concentrations were analyzed using an elemental analyzer (Vario ElIII, Elementar, Hanau, Germany) after air drying, sieving (2 mm mesh), and handpicking to remove surface vegetation and fine roots. Since inorganic C was not detected in any samples using a carbonate content analyzer (Eijkelkamp 08.53, Giesbeek, Netherlands), soil C was equal to organic C. The soil C:N ratio was calculated with organic C and total N. Soils collected with cutting rings to avoid compression were dried at 105 °C for 24 h to determine bulk density. Given that both residual vegetated patches and exposed soil patches were created by the abrupt thaw, we calculated the C stock loss within the collapse plots based on the weighted average of these two types of patches. Thus, the changes in soil C stock (0–15 cm) within the collapse plots (CSloss) were quantified following modified equations (2)–(4) (Abbott and Jones 2015):
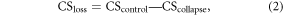


where CS is the C stock (kg C m−2), OC is the C concentration (g C kg−1 soil), BD is the bulk density (g cm−3), A is the percentage of feature area (%) and Depth is 15 cm.
Besides above-mentioned soil parameters, other soil properties (soil temperature, moisture, pH, δ13C and SOM composition) were determined for TS1, TS4, and TS6. Soil temperature was monitored in the field, where temperature sensors (DS 1922L, Wdsen Electronic Technology Co., Shanghai, China) were installed in collapse and control plots at 7.5 cm, and logged temperature every 2 h during the growing season from June to September in 2015. Gravimetric soil moisture was determined at the time of sampling by drying 20 g subsamples of fresh soil at 105 °C for 24 h. The pH of the soil suspension (1:5 soil–water ratio) was measured with a pH probe (PB-10, Sartorius, Germany). δ13C was analyzed with an elemental analyzer (Flash EA1112; Thermo Finnigan, Milan, Italy) coupled to an isotope ratio mass spectrometer (Finnigan MAT-253; Thermo Electron, Bremen, Germany) after soil samples being treated with 1 M HCl, washed with distilled water and oven dried at 65 °C for 24 h (Bird et al 1994). It has been used to reflect SOM decomposition due to the preferential utilization of the lighter isotope (12C) by soil microbes and consequent accumulation of 13C (Bird et al 1994, Strauss et al 2015).
2.4. SOM quality measurements
To determine the effects of permafrost collapse on SOM quality, we employed two molecular-level methods (i.e., 13C NMR and biomarker analysis) to determine the composition and degradation of SOM. In the NMR analysis, soil samples were repeatedly treated with 10% hydrofluoric acid to remove paramagnetic components, rinsed with deionized water and freeze-dried (Pautler et al 2010). Approximately 100 mg of treated samples was analyzed on a Bruker BioSpin Avance III 400 MHz WB spectrometer (Rheinstetten, Germany), equipped with a 4 mm standard bore CPMAS probe. The spin rate was 8 kHz, with a contact time of 2 ms and a 6 s recycle delay (Simpson and Simpson 2012). The spectra were integrated with the following chemical shift cutoffs: alkyl C (0–50 ppm), O-alkyl C (50–110 ppm), aromatic C (110–165 ppm), and carboxylic C (165–215 ppm) (Simpson and Simpson 2012). In the biomarker analysis, we employed sequential chemical extractions including solvent extraction, base hydrolysis and CuO oxidation to separate solvent-extractable compounds, hydrolysable lipids and lignin-derived phenols, respectively (Otto et al 2005). Details about biomarker analysis are provided in the supplementary methods. It has been widely demonstrated that O-alkyl C (mainly constituted by carbohydrates and peptides) is more labile to microbial decomposition than alkyl C (mainly constituted by lipids) (Pautler et al 2010, Simpson and Simpson 2012). Thus, in this study, we inferred O-alkyl C and carbohydrates as labile C fractions, and alkyl C and hydrolysable lipids (e.g. suberin and cutin) as recalcitrant C fractions.
2.5. Statistical analyses
We used linear mixed-effects models to test for differences in soil temperature, moisture, C stock, and SOM quality between collapse and control plots along the thaw sequence. In the mixed-effects analysis, plot location (collapse versus control) was included as a fixed effect and replicate as a random effect for each transect. Given that the sample size of original soil samples collected in different collapse plots was not identical, weighted least squares (WLS) regression analysis was performed to examine the relationship between changes in soil C concentration (ΔSOC) and years since collapse. The WLS regression analysis assumed that observations with larger weights provided more reliable information about the regression function than those with smaller weights. The sample size was used as the WLS weight in the analysis. In addition, ordinary least square regressions were performed to explore the relationship between soil C stock changes and years since collapse. All the statistical analyses were performed in R 3.2.4 (R Development Core Team, 2016) with the 'lme4' and 'stats' packages. Statistical differences were considered to be significant at the level of P < 0.05.
3. Results
3.1. Effects of abrupt permafrost thaw on microenvironments
Abrupt permafrost thaw fractured the intact swamp meadow into vegetated rafts (figures 1 and S1). The proportion of exposed soil was 11% at the early-stage site (1 year since collapse), increasing to 26% at the middle-stage site (10 years since collapse), and 35% at the late-stage site (16 years since collapse; table S1). Soil temperature in vegetated patches at 7.5 cm depth during the growing season was nearly 1 °C warmer than in control grassland in the middle stage of collapse (P = 0.015; table 1). Soil moisture in the thaw feature was lower than that in the control grassland in the early stage of collapse (P = 0.001), finally reaching 24.3% soil water loss in the late stage of collapse (P = 0.036; table 1).
Table 1. Soil characteristics in different years since collapse in an upland thaw feature on the Tibetan Plateau1.
| Years since collapse | Plot | Soil C stock (kg C m−2) | Soil N stock (kg N m−2) | Soil temperature (°C) | Soil moisture (wt%) | Soil pH | C:N | δ13C (‰) |
|---|---|---|---|---|---|---|---|---|
| 1 | Control | 6.47 (0.66) | 0.58 (0.04) | 8.7 (0.15) | 197.9 (6.1) | 5.72 (0.02) | 11.1 (0.21) | −24.7 (0.09) |
| Collapse | 6.25 (0.37) | 0.56 (0.05) | 8.8 (0.16) | 162.2 (4.5)* | 5.77 (0.03) | 11.2 (0.05) | −24.6 (0.05) | |
| 10 | Control | 6.24 (0.26) | 0.56 (0.03) | 8.6 (0.07) | 237.7 (12.4) | 5.66 (0.04) | 11.1 (0.06) | −24.6 (0.05) |
| Collapse | 4.57 (0.31)* | 0.40 (0.05)* | 9.5 (0.21)* | 199.3 (5.8)* | 5.71 (0.01) | 11.2 (0.05) | −24.6 (0.14) | |
| 16 | Control | 5.88 (0.54) | 0.52 (0.04) | 9.0 (0.09) | 220.1 (11.7) | 5.78 (0.04) | 11.3 (0.08) | −24.6 (0.07) |
| Collapse | 3.99 (0.75)* | 0.35 (0.07)* | 9.0 (0.18) | 166.7 (17.8)* | 5.77 (0.01) | 11.3 (0.06) | −24.8 (0.19) |
1Note that years since collapse were estimated through field observation and satellite imagery. Soil C and N stock were calculated with equations (2)–(4). The soil temperature presented here is the mean temperature of growing season from June to September in 2015. Gravimetric soil moisture was determined by dividing water weight by dry soil weight. Soil C:N was calculated with total organic C and total N. Standard errors are presented in parentheses. Significant differences between collapse and control plots are denoted by an asterisk (*) (P < 0.05).
3.2. Changes in soil C concentration and stock after permafrost collapse
We detected no change in soil C concentration (figure S4) and stock (figure 2(a)) during the first 7 years following collapse, but there was significant C loss at the middle and late stages (all P < 0.05; figures 2(a) and S4). Furthermore, the changes in soil C concentration and stock were negatively correlated with the time since collapse (all P < 0.05; figures 2(b)), with the maximum reduction of 30.7 g kg−1 and 1.89 kg C m−2 at the late stage. This amount of C stock loss in the topsoil (0–15 cm) accounts for more than 32% of the initial C stock (figure 2).
Figure 2. Changes in soil carbon (C) stock and concentration induced by permafrost collapse. (a) Comparison of soil C stock between collapse (red) and control (blue) plots along the thaw sequence. Notably, as the control plots (blue) were paired with the collapse plots (red), the variations of C stock in the control plots along the thaw sequence were due to spatial variability rather than abrupt thaw effects. (b) Relationships of changes in soil C stock (square) and C concentration (ΔSOC) (circle) with the years since collapse. The stand errors of slope and intercept in the C stock model are 0.02 and 0.18, respectively, while those in the C concentration model are 0.62 and 6.17, respectively. The negative values in panel (b) indicate C stock loss within the thaw feature. The size of the bubble is the relative weight of ΔSOC in the WLS regression. Larger bubbles indicate greater overall weight in the WLS regressions. The solid line is the regression curve, and the shaded area indicates the 95% confidence interval. Significant differences are denoted by an asterisk (*) (P < 0.05).
Download figure:
Standard image High-resolution image3.3. Changes in SOM quality along the thaw sequence
We observed no significant change in soil C:N ratio and δ13C after collapse (P > 0.05; table 1). However, the molecular-level (NMR and biomarker) approaches revealed substantial shifts in SOM composition (figures 3, 4 and S5). The NMR results revealed that the O-alkyl C shift region was abundant in all soil samples, with an average intensity of 43.8% along the thaw sequence (figure 3(b)). The collapsed soils showed a lower signal in the O-alkyl C region (3.2% decrease in easily degraded SOM constituents; P = 0.037), but a higher signal in the alkyl C region (12.2% increase in recalcitrant compounds; P = 0.019) than the control soil at the late stage of permafrost collapse (figures 3(a), (b)). Furthermore, the ratio of alkyl/O-alkyl was 15.8% higher in the collapsed soils than in the control at the late-stage site (P = 0.006; figure 3(d)), confirming a lower SOM quality compared to control after 16 years permafrost collapse.
Figure 3. Comparisons of SOM composition (measured by 13C NMR) between collapse (red) and control (blue) plots in different years since permafrost collapse (collapsed for 1, 10, and 16 years, respectively). Subpanels correspond to (a) alkyl C, (b) O-alkyl C, (c) aromatic C and (d) alkyl/O-alkyl ratio, respectively. The alkyl/O-alkyl ratio reflects the degree of SOM degradation (Simpson and Simpson 2012). Notably, as the control plots (blue) were paired with the collapse plots (red), the variations of SOM composition in the control plots along the thaw sequence were due to spatial variability rather than abrupt thaw effects. Error bars represent standard errors (n = 3). Significant differences are denoted by an asterisk (*) (P < 0.05).
Download figure:
Standard image High-resolution imageFigure 4. Comparisons of the relative abundance of major SOM components (measured by biomarker analysis) between collapse (red) and control (blue) plots in different years since permafrost collapse. Subpanels correspond to (a) carbohydrates, (b) suberin-derived compounds, and (c) lignin-derived phenols. Notably, as the control plots (blue) were paired with the collapse plots (red), the variations of SOM composition in the control plots along the thaw sequence were due to spatial variability rather than abrupt thaw effects. All values in the collapse plots are normalized against the corresponding values in the control plots. Error bars indicate standard errors (n = 3). Significant differences are denoted by an asterisk (*) (P < 0.05).
Download figure:
Standard image High-resolution imageThe biomarker analysis confirmed the pattern of an overall decrease in indicators of SOM quality at the late-stage site. The relative abundance of carbohydrates (trehalose) showed the greatest decrease (49.6%) at the late-stage site relative to the reference soil (P = 0.039; figure 4(a) and table S2). In contrast with the decrease in labile SOM fractions, suberin- and lignin-derived compounds increased by 29.0% (P = 0.017) and 15.3% (P = 0.04) (figure 4(b), (c)) at the late stage of permafrost collapse, respectively. Meanwhile, vanillic acid to vanillin (Ad/Al)v and syringic acid to syringaldehyde (Ad/Al)s, were similar between the different soils (table S3), further suggesting that the abrupt thaw could increase lignin phenol accumulation rather than degradation.
4. Discussion
4.1. Significant C losses induced by abrupt thaw
This study revealed that abrupt thaw in upland regions resulted in substantial soil C loss in a relatively short time (i.e., a 10% decrease in soil C concentration and 27% C stock loss 10 years after collapse) (figure 2), suggesting that abrupt thaw may be a dominant pathway of SOM mobilization and mineralization as high-latitude and high-altitude ecosystems experience faster climate warming (Harms et al 2014, Chipman et al 2016, Segal et al 2016, Jones et al 2017). This significant C loss caused by abrupt thaw contrasts sharply with the minor changes induced by gradual active-layer deepening based on the data from Circumpolar Active Layer Monitoring Network in Zackenberg (Elberling et al 2013). This difference may be explained by the different extents to which these two degradation pathways affect the local microhabitat. Compared with the gradual active-layer deepening, abrupt permafrost thaw may mobilize significant portions of the soil profile simultaneously (Abbott and Jones 2015), warming and draining soil, and disrupting plant growth (Perreault et al 2016, Jones et al 2017). Contrastingly, gradual thaw allows continued plant growth (i.e., C inputs) and even though newly thawed permafrost C is above freezing for a short period during maximum thaw depth, soil moisture remains high, impeding rapid mineralization (Elberling et al 2013, Schuur et al 2015). Consequently, vertical C loss in collapsed soil is generally higher than that in sites experiencing gradual thaw (Abbott and Jones 2015, Schuur et al 2015). Additionally, abrupt thaw can enhance lateral C export by increasing the area of soil exposure and breaking down soil structure (Abbott et al 2014, Liu et al 2018). Therefore, abrupt thaw would result in higher C loss than gradual active-layer deepening. While abrupt thaw features currently occupy less than 2% of the landscape, they may affect 20%–50% of uplands by the end of the century based on the distribution of ground ice and projections of permafrost degradation (Grosse et al 2011, Abbott and Jones 2015, Olefeldt et al 2016).
4.2. Decreased SOM quality after permafrost collapse
This study also demonstrated that labile carbon fractions (i.e., O-alkyl C in the NMR analysis and carbohydrates in the biomarker analysis) of SOM decreased whereas stable carbon fractions (i.e., alkyl C in the NMR analysis and suberin and lignin in the biomarker analysis) accumulated 16 years after permafrost collapse (figures 3 and 4). Notably, the aromatic C in the NMR analysis did not accumulate like lignin-derived phenols in the biomarker analysis, because a variety of components with different biodegradability (i.e., lignin-derived phenols, charcoal and methoxy in peptides) were included in the aromatic C (Pautler et al 2010, Simpson and Simpson 2012). Some components (i.e., methoxy in peptides) of aromatic C would be easily decomposed, while other components (i.e., lignin-derived phenols) were recalcitrant to degradation (Simpson and Simpson 2012). Collectively, these changes in SOM composition resulted in a decreased SOM quality after permafrost collapse, supporting our hypothesis. As depicted by the 13C NMR integration, easily degradable carbohydrates and peptides accounted for 43.8% of all SOM components (figure 3). After being exposed to aerobic conditions during permafrost collapse, these easily decomposable SOM can be rapidly decomposed by microorganisms (Pautler et al 2010, Mueller et al 2015). In contrast, suberin- and lignin-derived compounds have complex chemical structure (Otto et al 2005), making them resistant to microbial decomposition (Davidson and Janssens 2006). In addition, the hydrologic export of biodegradable compounds induced by abrupt thaw could also result in decreased quality of SOM (Abbott et al 2014, Vonk et al 2015), particularly since recalcitrant compounds are preferentially adsorbed to mineral soil (Kawahigashi et al 2006).
The decreasing indicators of quality observed in the Tibetan upland thaw feature contrast with recent observations that thaw-induced inundation in permafrost wetlands increases the abundance of biodegradable compounds (Hodgkins et al 2014). Such contradictory patterns highlight the effects of permafrost collapse on SOM quality may depend on site-specific environmental conditions and plant communities. Specifically, in wetlands, the thaw-induced inundation and consequent vegetation shift from moss to sedge led to an increase in SOM quality along a ∼40 years permafrost thaw progression (Hodgkins et al 2014). However, abrupt thaw in upland Tibetan regions did not cause vegetation succession at medium-term (∼20 years) timescales, but instead led to substantial changes in environmental conditions, which may change the SOM quality by affecting SOM decomposition. This finding was further supported by previous studies that showed increased microbial decomposition following permafrost collapse (Jorgenson et al 2013, Abbott and Jones 2015). Hence, the SOM quality would gradually decrease as indicated by our study, progressively slowing microbial activity—representing a potential stabilizing feedback for SOM exposed by permafrost collapse in upland ecosystems.
5. Conclusions and implications
This study provides evidence that abrupt thaw in upland regions triggers substantial soil C loss on decadal timescales. During this period, the labile SOM fractions (e.g. carbohydrates) significantly decreased while recalcitrant SOM fractions (e.g. suberin) increased, resulting in declined SOM quality. These findings have two important implications for understanding permafrost C-climate feedback. First, the significant decreases in soil C concentration and stock on decadal timescales contrast with observations that gradual thaw has little medium-term impact on soil C content (Elberling et al 2013). This difference highlights that while abrupt thaw features are currently spatially limited, they may exert stronger effects on medium-term soil C dynamics than distributed active layer deepening. Second, the observed decrease in SOM quality in dry, upland environments contrasted with the increased quality in wetland, inundated systems (Hodgkins et al 2014), suggesting that environmental conditions could exert major control over the quality of SOM exposed via permafrost collapse. Nevertheless, given that our results were only obtained from one typical abrupt thaw feature in the upland region of the Tibetan Plateau, further studies that include multiple features should be conducted to better understand spatial variations in abrupt thaw-induced soil C dynamics across Tibetan alpine permafrost region. Additionally, this study only focused on the topsoil (0–15 cm depth) C loss, the knowledge on the entire soil column including the permafrost layer is still limited, and future studies should thus pay more attention to changes in C stock within the entire soil column. Overall, the dynamic physical and microbial interactions following abrupt thaw highlight the importance of incorporating SOM quality, environmental conditions and other C loss processes into Earth System Models when predicting soil C dynamics.
Acknowledgments
This work was supported by the National Basic Research Program of China on Global Change (2014CB954001), National Natural Science Foundation of China (31670482 and 31400364), Key Research Program of Frontier Sciences, CAS (QYZDB-SSW-SMC049), and Chinese Academy of Sciences-Peking University Pioneer Cooperation Team. BWA was supported by the Department of Plant and Wildlife Sciences and College of Life Sciences at Brigham Young University. JS was supported by the European Research Council (Starting Grant #338335) and the Initiative and Networking Fund of the Helmholtz Association (#ERC-0013).





